| Pharmaceutical Information |
| Drug Name |
Saxagliptin |
| Drug ID |
BADD_D01992 |
| Description |
Saxagliptin (rINN) is an orally active hypoglycemic (anti-diabetic drug) of the new dipeptidyl peptidase-4 (DPP-4) inhibitor class of drugs. FDA approved on July 31, 2009. |
| Indications and Usage |
Treatment of type 2 diabetes mellitus to improve glycemic control in combination with other agents or as monotherapy. |
| Marketing Status |
Prescription |
| ATC Code |
A10BH03 |
| DrugBank ID |
DB06335
|
| KEGG ID |
D08996
|
| MeSH ID |
C502994
|
| PubChem ID |
11235729
|
| TTD Drug ID |
D0K9MY
|
| NDC Product Code |
0310-6105; 55154-6931; 11722-078; 0310-6100; 50370-0030; 50193-4212 |
| Synonyms |
saxagliptin | 3-hydroxyadamantylglycine-4,5-methanoprolinenitrile hydrate | Onglyza | BMS 477118 | BMS477118 | BMS-477118 |
|
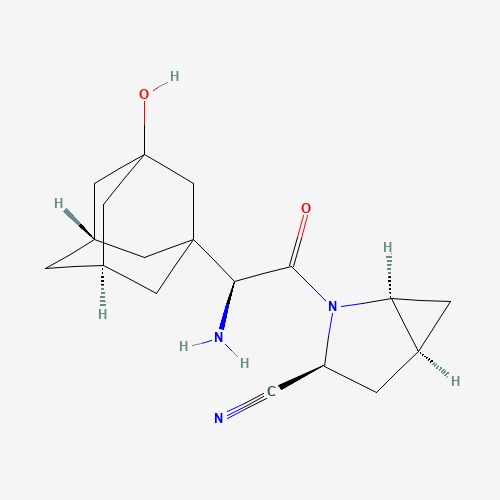
| Chemical Information |
| Molecular Formula |
C18H25N3O2 |
| CAS Registry Number |
361442-04-8 |
| SMILES |
C1C2CC2N(C1C#N)C(=O)C(C34CC5CC(C3)CC(C5)(C4)O)N |
| Chemical Structure |
|
|
| ADR Related Proteins Induced by Drug |
|
|
| ADRs Induced by Drug |
|
|

