| Pharmaceutical Information |
| Drug Name |
Pseudoephedrine hydrochloride |
| Drug ID |
BADD_D01877 |
| Description |
Pseudoephedrine is structurally related to [ephedrine] but exerts a weaker effect on the sympathetic nervous system.[A188820,A188823] Both drugs naturally occur in in ephedra plant which have a history of use in traditional Eastern medicine and were first researched in the west in 1889.[A188823] The decongestant effect of pseudoephedrine was described in dogs in 1927.[A188823] |
| Indications and Usage |
Pseudoephedrine is a sympathomimetic amine used for its decongestant activity.[L11031,L11037,L11040,L11046,L11052,L11058,L11061] |
| Marketing Status |
approved |
| ATC Code |
R01BA02 |
| DrugBank ID |
DB00852
|
| KEGG ID |
D00485
|
| MeSH ID |
D054199
|
| PubChem ID |
9581
|
| TTD Drug ID |
D00HHS
|
| NDC Product Code |
65498-363; 65724-4444; 30142-613; 36800-054; 49035-508; 51660-204; 51660-492; 70518-3405; 0904-6728; 51927-0125; 60646-102; 37808-007; 50580-536; 63187-541; 63868-143; 68196-204; 58747-7538; 21130-204; 41250-809; 70000-0475; 59779-703; 0363-3301; 0904-6907; 63552-075; 62011-0306; 10888-8168; 49452-6110; 65724-4400; 41163-054; 70000-0164; 70000-0601; 70518-2654; 24385-054; 50580-250; 65724-4401; 46122-166; 50090-4629; 56062-204; 68016-204; 0904-6727; 57218-801; 65724-4415; 0113-0054; 30142-967; 53943-204; 59779-204; 70677-0001; 0113-0401; 50580-242; 50580-363; 0363-0204; 51927-1159; 65724-4407; 50580-240 |
| UNII |
6V9V2RYJ8N
|
| Synonyms |
Pseudoephedrine | Pseudoephedrine Hydrochloride | Pseudoephedrine HCl | Sudafed | Isoephedrine | Threo Isomer of Ephedrine | Ephedrine Threo Isomer |
|
| Chemical Information |
| Molecular Formula |
C10H16ClNO |
| CAS Registry Number |
345-78-8 |
| SMILES |
CC(C(C1=CC=CC=C1)O)NC.Cl |
| Chemical Structure |
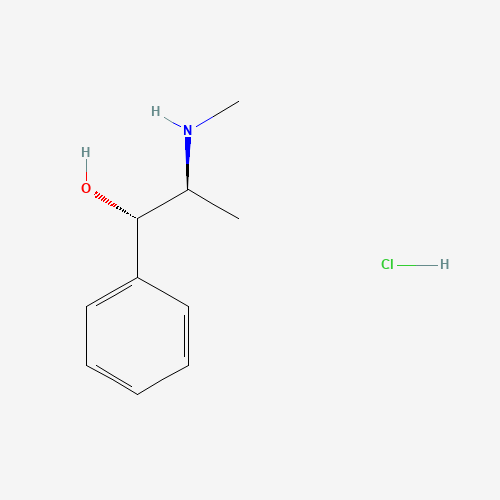
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Dizziness | 02.11.04.006; 24.06.02.007; 17.02.05.003 | - | - | | | Insomnia | 19.02.01.002; 17.15.03.002 | - | - | | | Nervousness | 19.06.02.003 | - | - | Not Available |
|
The 1th Page
1
Total 1 Pages
|
|

