| Pharmaceutical Information |
| Drug Name |
Pomalidomide |
| Drug ID |
BADD_D01798 |
| Description |
Pomalidomide, an analogue of thalidomide, is an immunomodulatory antineoplastic agent. FDA approved on February 8, 2013. |
| Indications and Usage |
Pomalidomide is indicated for patients with multiple myeloma who have received at least two prior therapies including lenalidomide and bortezomib and have demonstrated disease progression on or within 60 days of completion of the last therapy. |
| Marketing Status |
Prescription |
| ATC Code |
L04AX06 |
| DrugBank ID |
DB08910
|
| KEGG ID |
D08976
|
| MeSH ID |
C467566
|
| PubChem ID |
134780
|
| TTD Drug ID |
D0A3ZU
|
| NDC Product Code |
59572-504; 59572-503; 68554-0091; 82245-0108; 55111-989; 17337-0068; 65015-893; 68554-0092; 42973-232; 70225-1106; 54893-0038; 59572-501; 59572-502 |
| Synonyms |
pomalidomide | CC-4047 | CC 4047 | CC4047 | Imnovid | Pomalyst | actimid |
|
| Chemical Information |
| Molecular Formula |
C13H11N3O4 |
| CAS Registry Number |
19171-19-8 |
| SMILES |
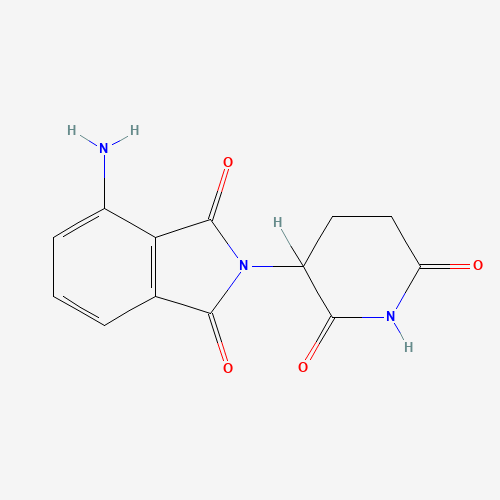
C1CC(=O)NC(=O)C1N2C(=O)C3=C(C2=O)C(=CC=C3)N |
| Chemical Structure |
|
|
| ADR Related Proteins Induced by Drug |
| ADR Term |
Protein Name |
UniProt AC |
TTD Target ID |
PMID |
| Not Available | Not Available | Not Available | Not Available | Not Available |
|
| ADRs Induced by Drug |
|
|

