| Pharmaceutical Information |
| Drug Name |
Polyethylene glycol 3350 |
| Drug ID |
BADD_D01793 |
| Description |
Polyethylene glycol (PEG) is a synthetic polymer produced via polymerization of ethylene oxide molecules to make joining units of ethylene glycol by an ether linkage.[A190975,A190978] PEGs are water-soluble polymers that can form hydrogen bonds in a ratio of 100 water molecules per one PEG molecule.[A190975] Molecular weights of PEGs vary by time of the polymerization process and the molecular weight represents the weighted average of the individual PEG molecules. PEGs differ in their physical and chemical properties depending on their molecular weight: PEGs are liquids when molecular weights are <1000 and the molecule turns to waxy solids with increasing molecular weights.[A190978] The most common preparations of PEGs include PEG 3350 and PEG 400. PEGs have various applications in many fields, ranging from medical to industrial areas. PEGs have a long history of gastroenterology: PEG 3350 is a common over-the-counter osmotic laxative used to relieve occasional constipation.[L11812] PEG 3350 is also used for cleansing of the colon in preparation for colonoscopy in adults.[L6421]
The rationale of using PEG in gastroenterology is due to the physical properties of the compound: its potent water-binding capacity, negligible intestinal absorption with increasing molecular mass, lack of significant toxicity, and limited intestinal enzymatic degradation or bacterial metabolism all make PEG a useful therapeutic agent for the treatment of occasional constipation and bowel cleansing for preparation in colonoscopy.[A18713] |
| Indications and Usage |
Polyethylene glycol is indicated for use as an over-the-counter osmotic laxative to relieve occasional constipation.[L11812] When used in combination with sodium ascorbate, sodium sulfate, ascorbic acid, sodium chloride and potassium chloride, it is used for cleansing of the colon in preparation for colonoscopy in adults.[L6421] |
| Marketing Status |
approved; vet_approved |
| ATC Code |
Not Available |
| DrugBank ID |
DB09287
|
| KEGG ID |
D03370
|
| MeSH ID |
C000595212
|
| PubChem ID |
174
|
| TTD Drug ID |
Not Available
|
| NDC Product Code |
79903-114; 43421-100; 57449-009; 59044-0005; 49348-143; 55154-2328; 58602-805; 59640-797; 69230-324; 69784-180; 11523-7268; 11534-180; 0113-0306; 30142-526; 0113-7306; 41250-306; 41520-306; 43386-312; 45802-868; 49348-893; 50594-306; 55154-8184; 55910-846; 57896-489; 68788-7508; 72288-020; 59044-0025; 11523-7234; 41163-306; 51991-962; 59779-306; 0363-0306; 70000-0415; 57896-490; 60687-431; 0363-0489; 63629-8541; 64024-306; 59044-0015; 49035-306; 50090-5310; 55301-306; 56062-306; 59556-762; 62559-157; 68391-306; 72288-499; 79481-0090; 51552-0088; 0113-6900; 37808-306; 53943-652; 63739-198; 59044-0019; 11673-306; 21130-847; 51991-961; 55319-306; 0363-1323; 63187-368; 63941-489; 68016-726; 0536-1052; 71932-000; 11523-4357; 11822-0306; 11822-1199; 0113-1023; 36800-306; 53943-742; 55154-8090; 55154-8091; 55910-306; 63868-002; 63981-306; 69256-306; 71205-447; 71204-100; 11523-7341; 62011-0287; 0904-6931; 71932-100; 46122-014; 50090-2430; 55154-5035; 62011-0153; 0363-5260; 68196-306; 69842-458; 30142-306; 49035-906 |
| UNII |
G2M7P15E5P
|
| Synonyms |
polyethylene glycol 3350 | PEG 3350 | miralax | SF-ELS | sulfate-free electrolyte lavage solution | NuLytely |
|
| Chemical Information |
| Molecular Formula |
C2H6O2 |
| CAS Registry Number |
107-21-1 |
| SMILES |
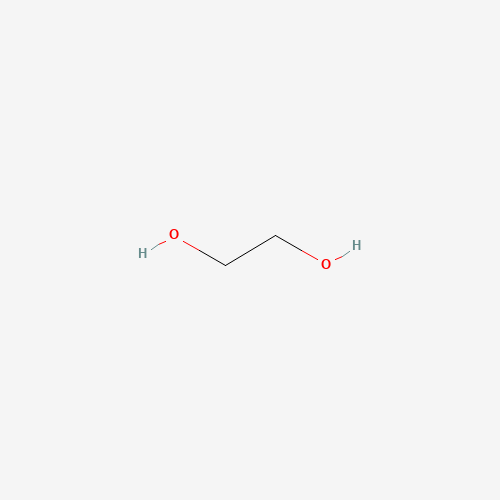
C(CO)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

