| Pharmaceutical Information |
| Drug Name |
Polidocanol |
| Drug ID |
BADD_D01792 |
| Description |
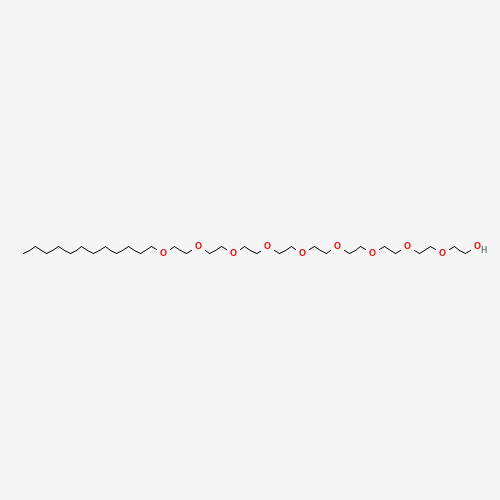
Polidocanol is a sclerosing agent indicated to treat uncomplicated spider veins (varicose veins ≤1 mm in diameter) and uncomplicated reticular veins (varicose veins 1 to 3 mm in diameter) in the lower extremity. It is marketed under the brand names Asclera and Varithena. The formula for Polidocanol has the structural formula C12H25(OCH2CH2)nOH, a mean extent of polymerization (n) of approximately 9 and a mean molecular weight of approximately 600. |
| Indications and Usage |
Polidocanol is a sclerosing agent indicated to treat uncomplicated spider veins and uncomplicated reticular veins in the lower extremity. |
| Marketing Status |
approved |
| ATC Code |
C05BB02 |
| DrugBank ID |
DB06811
|
| KEGG ID |
D01993
|
| MeSH ID |
D000077423
|
| PubChem ID |
656641
|
| TTD Drug ID |
D05ZPL
|
| NDC Product Code |
60635-107; 67850-140; 52919-021; 60635-111; 60635-118; 60635-133; 52919-024; 52919-022; 67850-141; 57821-011 |
| UNII |
0AWH8BFG9A
|
| Synonyms |
Polidocanol | Polidocanols | Laureth 9 | Laureth-9 | Lubrol 12A9 | Laureth-7 | Laureth 7 | Polyethylene Glycol-7-lauryl Ether | Ether, Polyethylene Glycol-7-lauryl | Polyethylene Glycol 7 lauryl Ether | Polyethylene Glycol-7-lauryl Ethers | Lauromacrogol | Lauromacrogols | Polyoxyethylene Lauryl Ether | Ether, Polyoxyethylene Lauryl | Polyoxyethylene Lauryl Ethers | Thesit | Nonaethylene Glycol Monododecyl Ether | Nonaethyleneglycol Monododecyl Ether | Ether, Nonaethyleneglycol Monododecyl | Monododecyl Ether, Nonaethyleneglycol | Nonaethyleneglycol Monododecyl Ethers | alpha-Dodecyl-omega-hydroxypoly(oxy-1,2ethanediyl) | Polyoxyethylene-4-dodecyl Ether | Ether, Polyoxyethylene-4-dodecyl | Polyoxyethylene 4 dodecyl Ether | Polyoxyethylenedodecyl Ether | Polyoxyethylenedodecyl Ethers | Tetraethyleneglycol Lauryl Ether | Ether, Tetraethyleneglycol Lauryl | Lauryl Ether, Tetraethyleneglycol | Tetraethyleneglycol Lauryl Ethers | Laureth-4 | Laureth 4 | Polyoxyethylene(4) Lauryl Ether | Lauromacrogol 400 | Polyethylene Glycol Monododecyl Ether | Brij 30 | Brij-30 | Brij30 | Tetraethylene Glycol Dodecyl Ether | Laureth | Laureths | Dodecyl Ethyleneglycol Monoether | Dodecyl Ethyleneglycol Monoethers | Ethyleneglycol Monoether, Dodecyl | Monoether, Dodecyl Ethyleneglycol | Laureth-1 | Laureth 1 | Aethoxysclerol | Ethoxysclerol | Aethoxysklerol | Atossisclerol | Atoxysclerol | Aetoxisclerol | Hydroxypolyethoxydodecane | Lubrol-PX | Lubrol PX | Polyoxyethylene 9-lauryl Ether | Ether, Polyoxyethylene 9-lauryl | Polyoxyethylene 9 lauryl Ether | Polyoxyethylene 9-lauryl Ethers |
|
| Chemical Information |
| Molecular Formula |
C30H62O10 |
| CAS Registry Number |
3055-99-0 |
| SMILES |
CCCCCCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Injection site discolouration | 23.03.03.046; 12.07.03.038; 08.02.03.038 | - | - | Not Available | | Nerve injury | 12.01.12.002; 17.02.10.007 | - | - | Not Available | | Hot flush | 24.03.01.005; 21.02.02.001; 08.01.03.027 | - | - | |
|
|
|

