| Pharmaceutical Information |
| Drug Name |
Pioglitazone |
| Drug ID |
BADD_D01777 |
| Description |
Pioglitazone is an antihyperglycemic used as an adjunct to diet, exercise, and other antidiabetic medications to manage type 2 diabetes mellitus.[L11416,L11419,L11422,L11425] It is administered as a racemic mixture, though there is no pharmacologic difference between the enantiomers and they appear to interconvert _in vivo_ with little consequence.[L11416] The thiazolidinedione class of medications, which also includes [rosiglitazone] and [troglitazone], exerts its pharmacological effect primarily by promoting insulin sensitivity and the improved uptake of blood glucose[L11416] via agonism at the peroxisome proliferator-activated receptor-gamma (PPARγ).[A19757] PPARs are ligand-activated transcription factors that are involved in the expression of more than 100 genes and affect numerous metabolic processes, most notably lipid and glucose homeostasis.[A19759]
Thiazolidinediones, including pioglitazone, have fallen out of favor in recent years due to the presence of multiple adverse effects and warnings regarding their use (e.g. congestive heart failure, bladder cancer) and the availability of safer and more effective alternatives for patients with type 2 diabetes mellitus.[L11461] |
| Indications and Usage |
Pioglitazone is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.[L11416] It is also available in combination with [metformin],[L11419] [glimepiride],[L11422] or [alogliptin][L11425] for the same indication. |
| Marketing Status |
approved; investigational |
| ATC Code |
A10BG03 |
| DrugBank ID |
DB01132
|
| KEGG ID |
D08378
|
| MeSH ID |
D000077205
|
| PubChem ID |
4829
|
| TTD Drug ID |
D03OFF
|
| NDC Product Code |
0093-7271; 50090-4586; 53002-2757; 61919-781; 64764-151; 68071-5210; 68382-308; 71335-1333; 71335-1385; 43547-428; 71610-495; 72189-105; 0781-5421; 0781-5422; 0904-7090; 50090-4556; 50090-6412; 53002-2755; 68071-5245; 68788-7514; 70518-2129; 71335-1759; 71335-1298; 16714-647; 33342-054; 57237-220; 57237-221; 68382-306; 68788-7989; 70518-2609; 70771-1021; 71335-1659; 0781-5420; 11532-0012; 16714-645; 0093-7272; 64764-301; 64764-451; 70771-1023; 71335-1343; 33342-055; 43547-427; 57237-219; 68071-2886; 68071-4964; 0904-7096; 16729-020; 50090-4605; 70518-2431; 70771-1022; 72189-293; 16714-646; 60687-391; 68788-7511; 71335-1137; 16729-021; 0093-7273; 43547-426; 68071-2264; 68382-307; 11532-0011; 11532-0013; 16729-022; 33342-056; 53002-2756 |
| UNII |
X4OV71U42S
|
| Synonyms |
Pioglitazone | 5-(4-(2-(5-Ethyl-2-pyridyl)ethoxy)benzyl)-2,4-thiazolidinedione | U 72107A | U72,107A | U-72107A | U72107A | AD 4833 | AD-4833 | AD4833 | Pioglitazone Hydrochloride | Actos |
|
| Chemical Information |
| Molecular Formula |
C19H20N2O3S |
| CAS Registry Number |
111025-46-8 |
| SMILES |
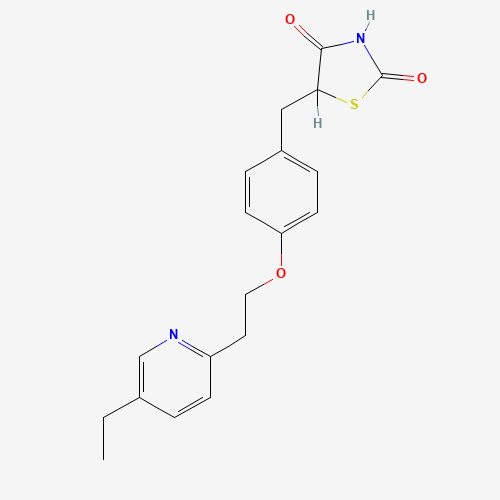
CCC1=CN=C(C=C1)CCOC2=CC=C(C=C2)CC3C(=O)NC(=O)S3 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
|
|
|

