| Pharmaceutical Information |
| Drug Name |
Oxymorphone |
| Drug ID |
BADD_D01648 |
| Description |
An opioid analgesic with actions and uses similar to those of morphine, apart from an absence of cough suppressant activity. It is used in the treatment of moderate to severe pain, including pain in obstetrics. It may also be used as an adjunct to anesthesia (From Martindale, The Extra Pharmacopoeia, 30th ed, p1092). On June 8, 2017, FDA requested Endo Pharmaceuticals to remove the medication from the market due to opioid misuse and abuse risks associated with the product's injectable reformulation. |
| Indications and Usage |
For the treatment of moderate-to-severe pain. |
| Marketing Status |
approved; investigational; vet_approved |
| ATC Code |
N02AA11 |
| DrugBank ID |
DB01192
|
| KEGG ID |
D08323
|
| MeSH ID |
D010111
|
| PubChem ID |
5284604
|
| TTD Drug ID |
D02NSF
|
| NDC Product Code |
0406-3433 |
| UNII |
9VXA968E0C
|
| Synonyms |
Oxymorphone | Oxymorphone Hydrochloride | Oxymorphone HCl | Numorphan | Opana |
|
| Chemical Information |
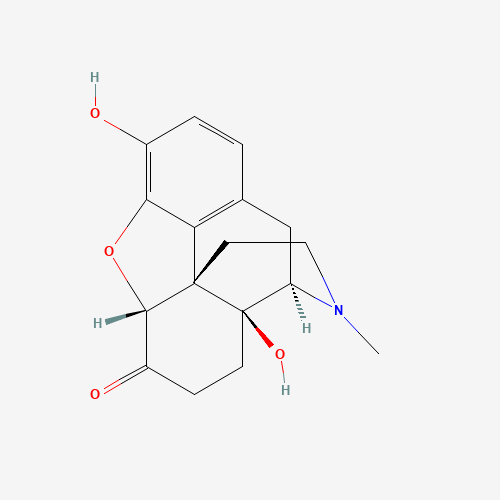
| Molecular Formula |
C17H19NO4 |
| CAS Registry Number |
76-41-5 |
| SMILES |
CN1CCC23C4C(=O)CCC2(C1CC5=C3C(=C(C=C5)O)O4)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

