| Pharmaceutical Information |
| Drug Name |
Morphine |
| Drug ID |
BADD_D01498 |
| Description |
Morphine, the main alkaloid of opium, was first obtained from poppy seeds in 1805.[A176035] It is a potent analgesic, though its use is limited due to tolerance, withdrawal, and the risk of abuse.[A176050] Morphine is still routinely used today, though there are a number of semi-synthetic opioids of varying strength such as [codeine], [fentanyl], [methadone], [hydrocodone], [hydromorphone], [meperidine], and [oxycodone].
Morphine was granted FDA approval in 1941.[L12114] |
| Indications and Usage |
Morphine is used for the management of chronic, moderate to severe pain.[A176050]
Opiods, including morphine, are effective for the short term management of pain. Patients taking opioids long term may need to be monitored for the development of physical dependence, addiction disorder, and drug abuse.[L5728] |
| Marketing Status |
approved; investigational |
| ATC Code |
N02AA01 |
| DrugBank ID |
DB00295
|
| KEGG ID |
D08233
|
| MeSH ID |
D009020
|
| PubChem ID |
5288826
|
| TTD Drug ID |
D0WE3O
|
| NDC Product Code |
42799-217 |
| UNII |
76I7G6D29C
|
| Synonyms |
Morphine | Morphia | Morphine Chloride | Chloride, Morphine | Morphine Sulfate | Sulfate, Morphine | SDZ 202-250 | SDZ 202 250 | SDZ 202250 | SDZ202-250 | SDZ202 250 | SDZ202250 | Morphine Sulfate (2:1), Pentahydrate | MS Contin | Contin, MS | Oramorph SR | Duramorph | Morphine Sulfate (2:1), Anhydrous |
|
| Chemical Information |
| Molecular Formula |
C17H19NO3 |
| CAS Registry Number |
57-27-2 |
| SMILES |
CN1CCC23C4C1CC5=C2C(=C(C=C5)O)OC3C(C=C4)O |
| Chemical Structure |
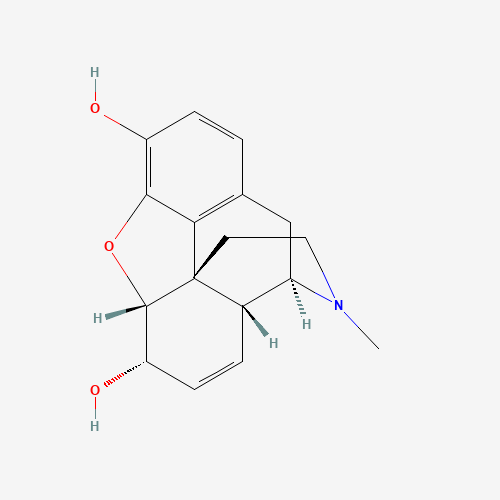
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Brain fog | 19.21.02.017; 17.02.05.077; 16.32.03.050 | - | - | Not Available | | Needle track marks | 23.03.11.045; 12.01.06.017 | - | - | Not Available | | Tissue rupture | 08.01.03.105 | - | - | Not Available |
|
|
|

