| Pharmaceutical Information |
| Drug Name |
Miltefosine |
| Drug ID |
BADD_D01469 |
| Description |
Miltefosine is a broad spectrum antimicrobial, anti-leishmanial, phospholipid drug that was originally developed in the 1980s as an anti-cancer agent. It is currently the only recognized oral agent used to treat visceral, cutaneous, and mucosal forms of leishmaniasis, a neglected tropical disease. It can be administered topically or orally and is only indicated in patients aged 12 years or older. The CDC has also recommended it as a first line treatment for free-living amebae (FLA) infections such as primary amebic meningoencephalitis and granulomatous amebic encephalitis. |
| Indications and Usage |
For the treatment of mucosal (caused by Leishmania braziliensis), cutaneous (caused by L. braziliensis, L. guyanensis, and L. panamensis), and visceral leishmaniasis (caused by L. donovani). In comparing Leishmania drug susceptibility, it has been found that L. donovani is the most susceptible to miltefosine while L. major is the least susceptible. Off-label use includes treatment of free-living amebae (FLA) infections (unlabeled use; CDC, 2013). |
| Marketing Status |
approved; investigational |
| ATC Code |
P01CX04 |
| DrugBank ID |
DB09031
|
| KEGG ID |
D02494
|
| MeSH ID |
C039128
|
| PubChem ID |
3599
|
| TTD Drug ID |
D00FGR
|
| NDC Product Code |
69051-300; 71052-509 |
| UNII |
53EY29W7EC
|
| Synonyms |
miltefosine | n-hexadecylphosphorylcholine | HDPC | hexadecylphosphocholine | Miltex | Impavido | D 18506 | D18506 | D-18506 |
|
| Chemical Information |
| Molecular Formula |
C21H46NO4P |
| CAS Registry Number |
58066-85-6 |
| SMILES |
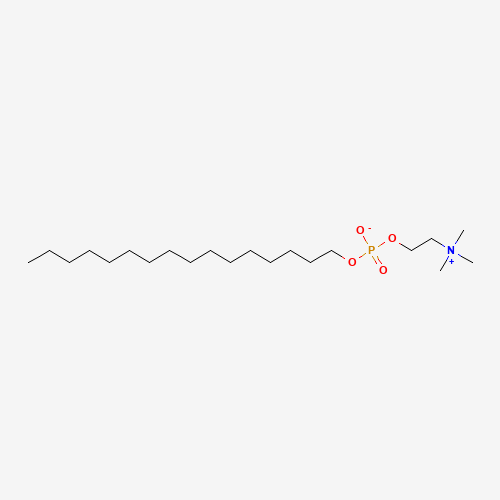
CCCCCCCCCCCCCCCCOP(=O)([O-])OCC[N+](C)(C)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

