| Pharmaceutical Information |
| Drug Name |
Mepact |
| Drug ID |
BADD_D01381 |
| Description |
Mifamurtide is an immunomodulator with antitumor activity via activation of macrophages and monocytes. Also called L-MTP-PE, mifamurtide may be a liposomal form of of the active ingredient MTP-PE, which is a synthetic, less pyrogenic, and longer-acting derivative of muramyl dipeptide (MDP). MDP is a motif present in all gram-positive and gram-negative bacterial walls that is recognized by different signalling molecules and activators such as nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs) and toll-like receptors present in macrophages and monocytes. The overall result of MDP recognition leads to the production of proinflammatory cytokines and promotion of bactericidal and tumoricidal effects [A31745]. As a liposomal formulation, mifamurtide demonstrates an enhanced tumoricidal effect and improved safety profile [A31745].
Mifamurtide is marketed in Europe as Mepact for intravenous infusion. It is administered as an adjuvant therapy to postoperative combination chemotherapy in pediatric, adolescent or adult patients with high-grade, resectable, non-metastatic osteosarcoma after macroscopically complete surgical resection. In the US, it is currently under investigation that holds orphan drug status for the treatment of osteosarcoma [A31746].
Osteosarcoma is the most common primary malignant bone tumor that usually arises in the metaphyses of long bone in children and adolescents [A31744]. The standard therapy for osteosarcoma is comprised of macroscopic surgical resection and multi-agent chemotherapy consisting of doxorubicin, cisplatin, high-dose methotrexate with leucovorin rescue, and ifosfamide [A31744]. While about 90% of patients with newly diagnosed osteosarcoma may achieve complete remission from first-line therapies, the prognosis is still poor for patients with non-metastatic osteosarcoma with lower 5-year event-free survival. In a large, randomized, open-label, multicenter, phase III trial, the treatment of mifamurtide in conjunction with three- or four-drug combination chemotherapy (doxorubicin, cisplatin, and high-dose methotrexate with, or without, ifosfamide) was associated with significant improvement in survival rates and good tolerance [A31746]. The adverse events (AEs) associated with mifamurtide were generally mild to moderate in severity [A31748]. |
| Indications and Usage |
Indicated in children, adolescents and young adults for the treatment of high-grade, resectable, non-metastatic osteosarcoma after macroscopically complete surgical resection, typically in combination with post-operative multi-agent chemotherapy [L1203]. |
| Marketing Status |
approved; experimental |
| ATC Code |
L03AX15 |
| DrugBank ID |
DB13615
|
| KEGG ID |
D06619
|
| MeSH ID |
C037144
|
| PubChem ID |
23663962
|
| TTD Drug ID |
D01NTX
|
| NDC Product Code |
Not Available |
| UNII |
Not Available
|
| Synonyms |
mifamurtide | muramyl tripeptide phosphatidylethanolamine | muramyl tripeptide-1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine conjugate | muramylNAc-Ala-isoGln-Lys-tripeptide-PE | muramylNAc-Ala-isoGln-Lys-tripeptide-phosphatidylethanolamine | N-acetylmuramyl-alanyl-isoglutaminyl-alanyl-sn-glycero-3-phosphoethanolamine | muramyl tripeptide-1,2-dipalmitoylphosphatidylethanolamine conjugate | MLV 19835 | Mepact | muramyl tripeptide phosphatidylethanolamine, monosodium salt | mifamurtide sodium | mifamurtide anhydrous | mifamurtide sodium salt | muramyl tripeptide phosphatidylethanolamine, sodium salt | CGP 19835 A | CGP-19835A | mifamurtide acid | mifamurtide free acid anhydrous |
|
| Chemical Information |
| Molecular Formula |
C59H110N6NaO20P |
| CAS Registry Number |
838853-48-8 |
| SMILES |
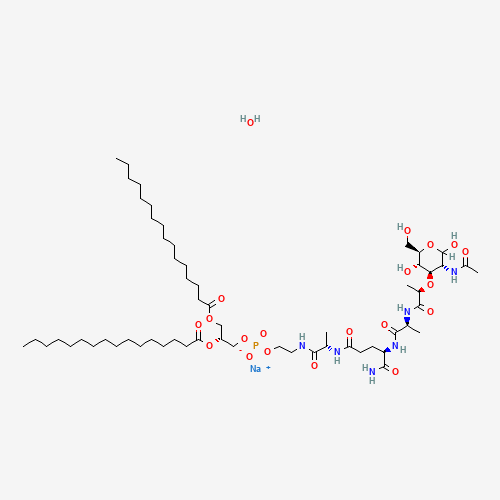
CCCCCCCCCCCCCCCC(=O)OCC(COP(=O)([O-])OCCNC(=O)C(C)NC(=O)CCC(C(=O)N)NC(=O)C(C)NC(
=O)C(C)OC1C(C(OC(C1O)CO)O)NC(=O)C)OC(=O)CCCCCCCCCCCCCCC.O.[Na+] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

