| Pharmaceutical Information |
| Drug Name |
Macitentan |
| Drug ID |
BADD_D01336 |
| Description |
Macitentan is a dual endothelin receptor antagonist used in the treatment of pulmonary arterial hypertension.[L35890] It was first approved by the FDA in 2013. Macitentan differs from its predecessor [bosentan] due to its lower risk of hepatotoxicity. |
| Indications and Usage |
Macitentan is indicated for patients with pulmonary arterial hypertension. |
| Marketing Status |
Prescription |
| ATC Code |
C02KX04 |
| DrugBank ID |
DB08932
|
| KEGG ID |
D10135
|
| MeSH ID |
C533860
|
| PubChem ID |
16004692
|
| TTD Drug ID |
D0S7JH
|
| NDC Product Code |
11722-058; 65015-872; 69766-006; 68225-070; 82245-0117; 69037-0030; 66215-501; 76397-014; 46708-893; 59651-122 |
| Synonyms |
macitentan | N-(5-(4-bromophenyl)-6-(2-(5-bromopyrimidin-2-yloxy)ethoxy)pyrimidin-4-yl)-N'-propylaminosulfonamide | ACT 064992 | ACT064992 | ACT-064992 | Actelion-1 | opsumit |
|
| Chemical Information |
| Molecular Formula |
C19H20Br2N6O4S |
| CAS Registry Number |
441798-33-0 |
| SMILES |
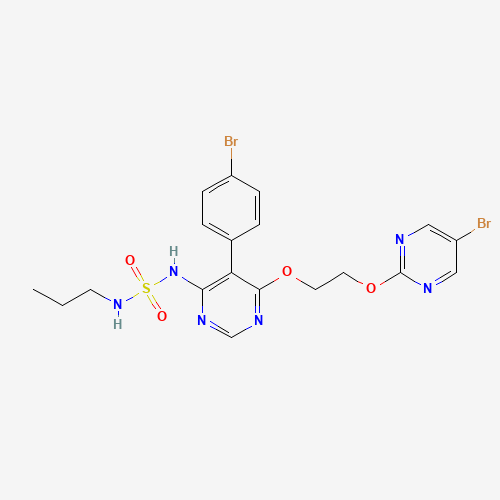
CCCNS(=O)(=O)NC1=C(C(=NC=N1)OCCOC2=NC=C(C=N2)Br)C3=CC=C(C=C3)Br |
| Chemical Structure |
|
|
| ADR Related Proteins Induced by Drug |
| ADR Term |
Protein Name |
UniProt AC |
TTD Target ID |
PMID |
| Not Available | Not Available | Not Available | Not Available | Not Available |
|
| ADRs Induced by Drug |
|
|

