| Pharmaceutical Information |
| Drug Name |
Luliconazole |
| Drug ID |
BADD_D01329 |
| Description |
Luliconazole is a topical antifungal agent that acts by unknown mechanisms but is postulated to involve altering the synthesis of fungi cell membranes. It was approved by the FDA (USA) in November 2013 and is marketed under the brand name Luzu. Luliconazole is also approved in Japan. |
| Indications and Usage |
Luliconazole is indicated in adults aged 18 years and older for the topical treatment of fungal infections caused by Trichophyton rubrum and Epidermophyton floccosum, specifically tinea pedis, cruris, and corporis.
|
| Marketing Status |
approved |
| ATC Code |
D01AC18 |
| DrugBank ID |
DB08933
|
| KEGG ID |
D01980
|
| MeSH ID |
C112528
|
| PubChem ID |
3003141
|
| TTD Drug ID |
D0U4XJ
|
| NDC Product Code |
70600-009; 99207-850; 68682-850; 66039-918 |
| UNII |
RE91AN4S8G
|
| Synonyms |
luliconazole | (2E)-((4R)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene)(1H-imidazol-1-yl)acetonitrile | 4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene-1-imidazolylacetonitrile | Lulicon | NND 502 | NND502 | NND-502 |
|
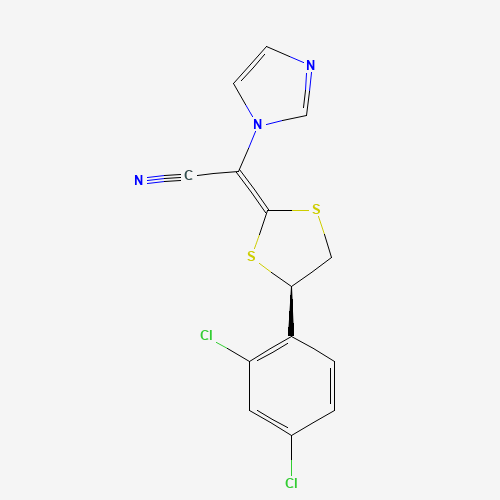
| Chemical Information |
| Molecular Formula |
C14H9Cl2N3S2 |
| CAS Registry Number |
187164-19-8 |
| SMILES |
C1C(SC(=C(C#N)N2C=CN=C2)S1)C3=C(C=C(C=C3)Cl)Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Application site reaction | 08.02.01.006; 12.07.01.006 | - | - | Not Available | | Cellulitis | 23.11.02.004; 11.02.01.001 | - | - | Not Available | | Dermatitis contact | 23.03.04.004; 12.03.01.040; 10.01.01.003 | - | - | Not Available | | Tinea cruris | 23.11.03.010; 11.03.08.001 | - | - | Not Available | | Tinea pedis | 23.11.03.012; 11.03.08.004 | - | - | Not Available |
|
The 1th Page
1
Total 1 Pages
|
|

