| Pharmaceutical Information |
| Drug Name |
Liothyronine sodium |
| Drug ID |
BADD_D01297 |
| Description |
Liothyronine is a thyroidal hormone T3 which is normally produced by the thyroid gland in a ratio 4:1 when compared with T4: T3. Liothyronine is the active form of thyroxine which is composed in a basic chemical structure by a tyrosine with bound iodine.[T457] The exogenous liothyronine product was developed by King Pharmaceuticals and FDA approved in 1956.[L5578] |
| Indications and Usage |
Liothyronine is officially approved for the following indications:
- Replacement therapy in primary (thyroidal), secondary (pituitary) and tertiary (hypothalamic) congenital or acquired hypothyroidism.
- As an adjunct therapy to surgery and radioiodine in the management of thyroid cancer.
- As a diagnostic agent in suppression tests for mild hyperthyroidism or thyroid gland autonomy.[FDA label]
In general terms, exogenous liothyronine is used to replace insufficient hormonal production and restore T3 plasma levels.[T457]
The lack of liothyronine can be presented as a pale and puffy face, coarse, brittle hair, dry skin, croaky voice and constipation as well as irregular periods, drowsiness, and lethargy.[T457]
Liothyronine should never be used in the suppression of benign nodules and nontoxic diffuse goiter in iodine-sufficient patients nor in the treatment of hyperthyroidism during the recovery phase of subacute thyroiditis.[FDA label] |
| Marketing Status |
approved; vet_approved |
| ATC Code |
H03AA02 |
| DrugBank ID |
DB00279
|
| KEGG ID |
D01011
|
| MeSH ID |
D014284
|
| PubChem ID |
23666110
|
| TTD Drug ID |
D0S6JG
|
| NDC Product Code |
16714-166; 42291-418; 51407-385; 70771-1606; 38779-0031; 51552-0354; 16714-167; 62756-591; 71335-0166; 62991-1096; 42291-419; 51862-320; 60793-117; 71205-150; 71335-1671; 71335-1943; 59762-1206; 62756-590; 63629-8337; 68382-582; 70771-1607; 67891-003; 43742-0956; 50090-5586; 68382-583; 42794-020; 51862-322; 59762-1207; 62756-589; 68788-7269; 71335-1978; 51927-0282; 16714-168; 0093-2179; 42794-018; 71335-0341; 71335-2001; 39822-0151; 42794-019; 43063-884; 59762-1208; 68382-584; 70771-1608; 71335-9632; 38779-3226; 42291-417; 51407-384; 51862-321; 60793-115; 60793-116; 69575-4010; 0093-2178; 0093-2180 |
| UNII |
GCA9VV7D2N
|
| Synonyms |
Triiodothyronine | T3 Thyroid Hormone | Thyroid Hormone, T3 | Liothyronine | 3,3',5-Triiodothyronine | Liothyronine Sodium | Cytomel |
|
| Chemical Information |
| Molecular Formula |
C15H11I3NNaO4 |
| CAS Registry Number |
55-06-1 |
| SMILES |
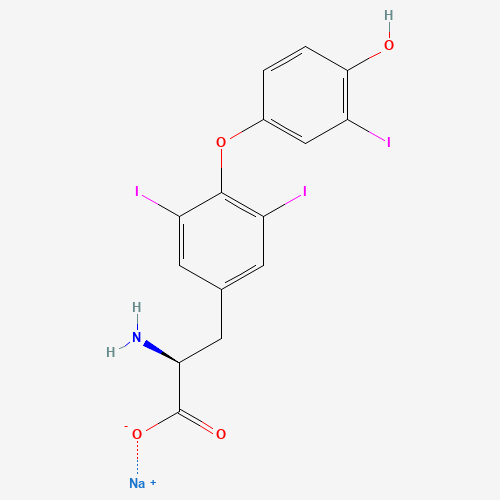
C1=CC(=C(C=C1OC2=C(C=C(C=C2I)CC(C(=O)[O-])N)I)I)O.[Na+] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Dermatitis allergic | 23.03.04.003; 10.01.03.014 | - | - | Not Available |
|
The 1th Page
1
Total 1 Pages
|
|

