| Pharmaceutical Information |
| Drug Name |
Linaclotide |
| Drug ID |
BADD_D01291 |
| Description |
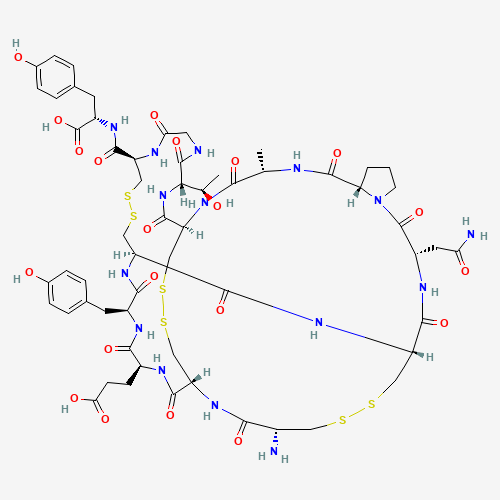
Linaclotide is an orally administered, peptide agonist of guanylate cyclase 2C for the treatment of irritable bowel syndrome. Chemically, it is a heterodetic cyclic peptide and consists of fourteen amino acids. The protein sequence is as follows: Cys Cys Glu Tyr Cys Cys Asn Pro Ala Cys Thr Gly Cys Tyr. There are three disulfide bonds which are located between Cys1 and Cys6; between Cys2 and Cys10; and between Cys5 and Cys13. FDA approved on August 30, 2012. |
| Indications and Usage |
Treatment of irritable bowel syndrome (IBS) with constipation and chronic idiopathic constipation. |
| Marketing Status |
approved |
| ATC Code |
A06AX04 |
| DrugBank ID |
DB08890
|
| KEGG ID |
D09355
|
| MeSH ID |
C523483
|
| PubChem ID |
16158208
|
| TTD Drug ID |
D00GNJ
|
| NDC Product Code |
59651-011; 0456-1204; 0456-1203; 14403-0014; 0456-1202; 69766-007; 0456-1206; 12869-110; 63557-0014; 0456-1205; 68625-121; 68625-122; 0456-1201; 32861-0010 |
| UNII |
N0TXR0XR5X
|
| Synonyms |
linaclotide | ASP-0456 | ASP0456 | Linzess | MD-1100 | Linaclotide Acetate | MD-1100 acetate |
|
| Chemical Information |
| Molecular Formula |
C59H79N15O21S6 |
| CAS Registry Number |
851199-59-2 |
| SMILES |
CC1C(=O)NC2CSSCC3C(=O)NC(C(=O)NC(C(=O)NC(CSSCC(NC(=O)CNC(=O)C(NC2=O)C(C)O)C(=O)N
C(CC4=CC=C(C=C4)O)C(=O)O)C(=O)NC(CSSCC(C(=O)N3)N)C(=O)NC(C(=O)N5CCCC5C(=O)N1)CC(
=O)N)CC6=CC=C(C=C6)O)CCC(=O)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

