| Pharmaceutical Information |
| Drug Name |
Levofloxacin |
| Drug ID |
BADD_D01275 |
| Description |
Levofloxacin is a fluoroquinolone antibiotic and the optical S-(-) isomer of racemic [ofloxacin].[A190663] It reportedly carries 8 to 128-fold more activity against both gram-negative and gram-positive bacteria compared to R-(+)-ofloxacin[A190663] and remains stereochemically stable following administration (i.e. it does not invert to the inactive isomer).[L11638] Levofloxacin, along with other quinolones such as [gatifloxacin] and [moxifloxacin], is a member of the third generation of fluoroquinolones, colloquially referred to as the "respiratory quinolones" due to improved activity against gram-positive bacteria commonly implicated in respiratory infections.[A31453,A190756]
Levofloxacin was first approved by the FDA in 1996, and was approved in Canada and several South American countries soon after.[A190663] |
| Indications and Usage |
In oral and intravenous formulations, levofloxacin is indicated in adults for the treatment of various infections caused by susceptible bacteria, including infections of the upper respiratory tract, lower respiratory tract, skin, skin structures, urinary tract, and prostate.[L11638,L11692] The oral formulation is also indicated in both adults and children 6 months of age and older for the post-exposure management of inhalational anthrax caused by _Bacillus anthracis_ and for the treatment and/or prophylaxis of plague caused by _Yersinia pestis_.[L11638]
In its ophthalmic formulation, levofloxacin is indicated for the treatment of bacterial conjunctivitis caused by susceptible organisms.[L11641] An inhalational solution available in Canada is indicated for the management of cystic fibrosis patients aged 18 years or older with chronic pulmonary _Pseudomonas aeruginosa_ infections.[L11689] |
| Marketing Status |
approved; investigational |
| ATC Code |
J01MA12; S01AE05 |
| DrugBank ID |
DB01137
|
| KEGG ID |
D00588; D08120
|
| MeSH ID |
D064704
|
| PubChem ID |
149096
|
| TTD Drug ID |
D02RSN
|
| NDC Product Code |
0904-6353; 31722-723; 33342-532; 36000-294; 43063-457; 43063-637; 54288-140; 63187-004; 63187-384; 63187-438; 63187-514; 65841-693; 67296-1546; 70518-0968; 70518-3712; 72189-338; 0904-6352; 49587-102; 17478-107; 36000-045; 45865-758; 55154-5898; 63187-833; 63323-355; 68788-8340; 71335-0434; 72578-098; 0781-5791; 80425-0007; 80425-0008; 80425-0009; 82982-034; 53104-7550; 63415-0056; 33342-022; 36000-296; 0143-9720; 50090-4572; 51655-666; 61919-440; 61919-704; 67296-0744; 67296-0897; 67296-1393; 0409-0528; 70771-1079; 71205-058; 55111-281; 65841-691; 65862-537; 68083-416; 68180-242; 68382-989; 69097-287; 0527-1948; 70518-0453; 71335-0794; 53069-0600; 65862-488; 31722-721; 33342-021; 0143-9317; 43063-638; 67296-0972; 67296-0977; 67296-1400; 68071-3236; 68083-415; 70518-0719; 71335-0593; 72578-099; 0904-6351; 65862-536; 0143-9316; 50383-286; 70934-094; 71335-1208; 65862-538; 16571-150; 25021-132; 33342-023; 33342-531; 36000-048; 44567-436; 50090-3314; 51655-947; 55111-279; 63187-925; 67296-1325; 67296-1351; 67296-1535; 68071-4512; 68083-395; 68180-241; 70518-0587; 0781-5790; 0781-5792; 43235-0007; 31722-722; 36000-046; 43063-796; 0143-9721; 51407-428; 67296-0975; 67296-0985; 71205-002; 71205-234; 71335-0380; 72789-085; 72789-248; 17478-106; 0143-9315; 44567-437; 50090-4797; 53002-1350; 55111-280; 55150-156; 55150-157; 63187-003; 68083-414; 68788-6938; 72578-100; 80425-0077; 55525-0001; 36000-295; 44567-435; 50090-3214; 50090-3311; 51407-429; 51655-327; 61919-426; 65841-692; 68083-394; 68180-240; 80425-0076; 65977-0039; 33342-533; 36000-047; 42571-168; 43063-459; 0143-9722; 51407-430; 53002-2350; 55154-5897; 55700-729 |
| UNII |
6GNT3Y5LMF
|
| Synonyms |
Levofloxacin | Ofloxacin, (S)-Isomer | Levofloxacin Anhydrous | Anhydrous, Levofloxacin | Quixin | Levaquin |
|
| Chemical Information |
| Molecular Formula |
C18H20FN3O4 |
| CAS Registry Number |
100986-85-4 |
| SMILES |
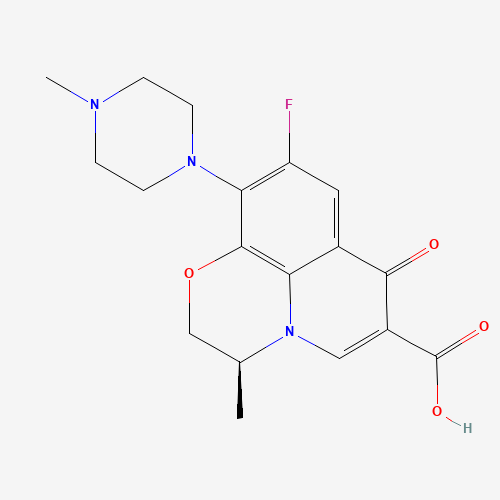
CC1COC2=C3N1C=C(C(=O)C3=CC(=C2N4CCN(CC4)C)F)C(=O)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Brain fog | 17.02.05.077; 16.32.03.050; 19.21.02.017 | - | - | Not Available |
|
|
|

