| Pharmaceutical Information |
| Drug Name |
Levobupivacaine |
| Drug ID |
BADD_D01269 |
| Description |
Levobupivacaine is an amino-amide local anaesthetic drug belonging to the family of n-alkylsubstituted
pipecoloxylidide. It is the S-enantiomer of bupivacaine. Levobupivacaine hydrochloride is commonly marketed by AstraZeneca under the trade name Chirocaine. In particular, the specific levobupivacaine enantiomer is a worthwhile pursuit because it demonstrates less vasodilation and possesses a greater length of action in comparison to bupivacaine. It is approximately 13 per cent less potent (by molarity) than racemic bupivacaine.Levobupivacaine is indicated for local anaesthesia including infiltration, nerve block, ophthalmic, epidural and intrathecal anaesthesia in adults; and infiltration analgesia in children. When administered appropriately, the occurrence of adverse effects is not anticipated much if at all. In general, the majority of potential adverse effects are predominantly associated with inappropriate administration methods that may cause systemic exposure and/or toxicity associated with overexposure to an anesthetic. Regardless, allergic reactions may also occur - although only rarely. |
| Indications and Usage |
For the production of local or regional anesthesia for surgery and obstetrics, and for post-operative pain management |
| Marketing Status |
approved; investigational |
| ATC Code |
N01BB10 |
| DrugBank ID |
DB01002
|
| KEGG ID |
D08116
|
| MeSH ID |
D000077554
|
| PubChem ID |
92253
|
| TTD Drug ID |
D09QUQ
|
| NDC Product Code |
61876-0728 |
| UNII |
A5H73K9U3W
|
| Synonyms |
Levobupivacaine | Levobupivacaine Hydrochloride | Chirocaine |
|
| Chemical Information |
| Molecular Formula |
C18H28N2O |
| CAS Registry Number |
27262-47-1 |
| SMILES |
CCCCN1CCCCC1C(=O)NC2=C(C=CC=C2C)C |
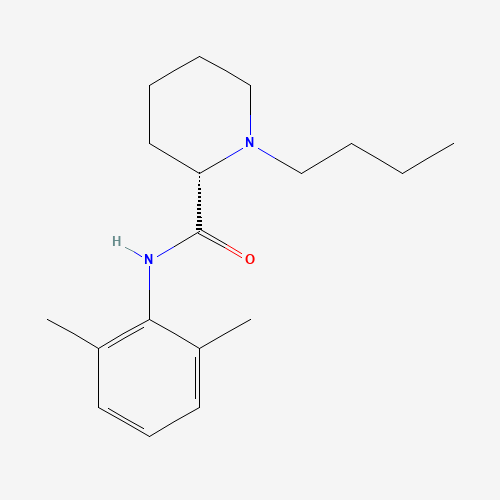
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

