| Pharmaceutical Information |
| Drug Name |
Lenalidomide |
| Drug ID |
BADD_D01253 |
| Description |
Lenalidomide (previously referred to as CC-5013) is an immunomodulatory drug with potent antineoplastic, anti-angiogenic, and anti-inflammatory properties.[A228553] It is a 4-amino-glutamyl analogue of [thalidomide] [A228543] and like thalidomide, lenalidomide exists as a racemic mixture of the active S(-) and R(+) forms.[A228708] However, lenalidomide is much safer and potent than thalidomide, with fewer adverse effects and toxicities.[A714, A228543] Thalidomide and its analogues, including lenalidomide, are referred to as immunomodulatory imide drugs (also known as cereblon modulators), which are a class of immunomodulatory drugs that contain an imide group. Lenalidomide works through various mechanisms of actions that promote malignant cell death and enhance host immunity.[A228708] Available as oral capsules, lenalidomide is approved by the FDA and EU for the treatment of multiple myeloma, myelodysplastic syndromes, mantle cell lymphoma, follicular lymphoma, and marginal zone lymphoma in selected patients.[L16028] Lenalidomide is available only under a special restricted distribution program.[A228708] |
| Indications and Usage |
Lenalidomide is indicated for the treatment of adult patients with multiple myeloma (MM) in combination with dexamethasone. It is also indicated as maintenance therapy in multiple myeloma following autologous hematopoietic stem cell transplantation (auto-HSCT).
It is indicated for the treatment of adult patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes (MDS) associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities.
Lenalidomide is indicated for the treatment of adult patients with mantle cell lymphoma (MCL) whose disease has relapsed or progressed after two prior therapies, one of which included bortezomib.
In combination with a rituximab product, lenalidomide is indicated for the treatment of adult patients with previously treated follicular lymphoma (FL) or previously treated marginal zone lymphoma (MZL).[L16028] |
| Marketing Status |
approved |
| ATC Code |
L04AX04 |
| DrugBank ID |
DB00480
|
| KEGG ID |
D04687
|
| MeSH ID |
D000077269
|
| PubChem ID |
216326
|
| TTD Drug ID |
D0Q5NX
|
| NDC Product Code |
54893-0061; 65015-772; 82991-402; 31722-257; 43598-512; 59572-410; 59572-425; 60505-4534; 0378-1941; 70710-1033; 53104-7720; 68554-0090; 82245-0101; 43598-516; 47781-486; 63304-044; 0378-1936; 70710-1032; 70710-1034; 70710-1035; 14096-131; 55111-911; 59651-733; 31722-260; 31722-262; 43598-514; 43598-515; 59572-420; 59651-342; 63304-042; 63304-043; 70771-1676; 0378-1935; 0480-1241; 0480-1242; 65727-084; 71796-026; 31722-261; 43598-511; 47781-484; 47781-487; 47781-488; 59572-405; 59651-346; 59651-347; 63304-045; 0378-1937; 0480-1246; 70771-1681; 0378-1942; 0480-1244; 70710-1031; 76282-697; 76282-699; 76282-701; 59116-4710; 31722-258; 59572-402; 59651-344; 0378-1940; 69097-604; 0480-1245; 70710-1030; 76282-698; 43598-513; 47781-483; 59572-415; 59651-345; 60505-4533; 63304-041; 69097-381; 69097-382; 69097-383; 69097-384; 69097-385; 70771-1680; 59651-343; 60505-4536; 63304-046; 0480-1243; 70771-1679; 55488-0700; 47781-485; 60505-4532; 60505-4535; 60505-4537; 70771-1677; 70771-1678; 53104-7726; 31722-259 |
| UNII |
F0P408N6V4
|
| Synonyms |
Lenalidomide | 3-(4-Amino-1-oxoisoindolin-2-yl)piperidine-2,6-dione | 2,6-Piperidinedione, 3-(4-amino-1,3-dihydro-1-oxo-2H- isoindol-2-yl)- | IMiD3 Cpd | CC 5013 | CC5013 | CC-5013 | Revlimid | Revimid |
|
| Chemical Information |
| Molecular Formula |
C13H13N3O3 |
| CAS Registry Number |
191732-72-6 |
| SMILES |
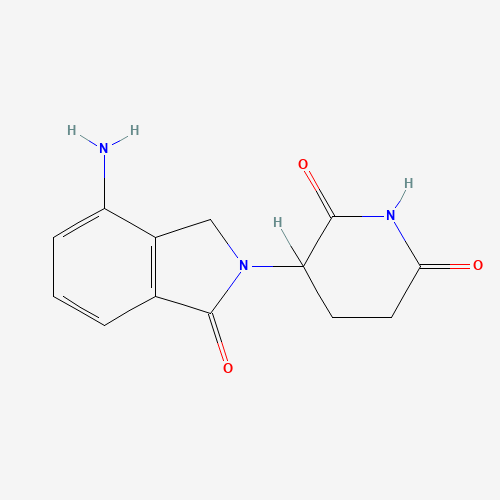
C1CC(=O)NC(=O)C1N2CC3=C(C2=O)C=CC=C3N |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
|
|
|

