| Pharmaceutical Information |
| Drug Name |
Hydroxychloroquine |
| Drug ID |
BADD_D01103 |
| Description |
Hydroxychloroquine is a racemic mixture consisting of an R and S enantiomer.[A183047] Hydroxychloroquine is an aminoquinoline like [chloroquine].[L8072] It is a commonly prescribed medication in the treatment of uncomplicated malaria, rheumatoid arthritis, chronic discoid lupus erythematosus, and systemic lupus erythematosus.[L8072] Hydroxychloroquine is also used for the prophylaxis of malaria in regions where chloroquine resistance is unlikely.[L8072] It was developed during World War II as a derivative of [quinacrine] with less severe side effects.[A183092] Chloroquine and hydroxychloroquine are both being investigated for the treatment of SARS-CoV-2.[A192132]
**The FDA emergency use authorization for hydroxychloroquine and [chloroquine] in the treatment of COVID-19 was revoked on 15 June 2020.[L14312]**
Hydroxychloroquine was granted FDA approval on 18 April 1955.[L8072]
A recent study reported a fatality in the group being treated with hydroxychloroquine for COVID-19.[A192546] |
| Indications and Usage |
Hydroxychloroquine is indicated for the prophylaxis of malaria where chloroquine resistance is not reported, treatment of uncomplicated malaria (caused by _P. falciparum_, _P. malariae_, _P. ovale_, or _P. vivax_), chronic discoid lupus erythematosus, systemic lupus erythematosus, acute rheumatoid arthritis, and chronic rheumatoid arthritis.[L8072] |
| Marketing Status |
approved |
| ATC Code |
P01BA02 |
| DrugBank ID |
DB01611
|
| KEGG ID |
D08050
|
| MeSH ID |
D006886
|
| PubChem ID |
3652
|
| TTD Drug ID |
D0OJ4L
|
| NDC Product Code |
Not Available |
| UNII |
4QWG6N8QKH
|
| Synonyms |
Hydroxychloroquine | Oxychlorochin | Oxychloroquine | Hydroxychlorochin | Plaquenil | Hydroxychloroquine Sulfate | Hydroxychloroquine Sulfate (1:1) Salt |
|
| Chemical Information |
| Molecular Formula |
C18H26ClN3O |
| CAS Registry Number |
118-42-3 |
| SMILES |
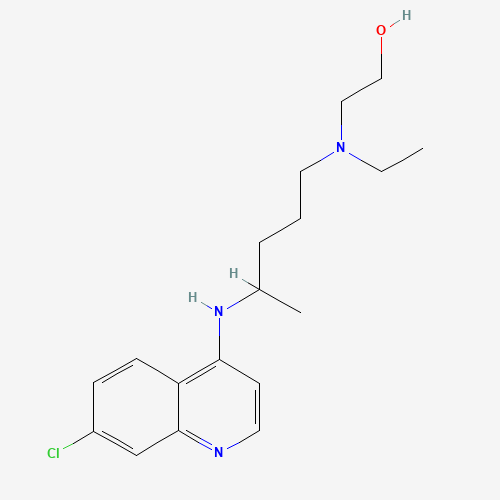
CCN(CCCC(C)NC1=C2C=CC(=CC2=NC=C1)Cl)CCO |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

