| Pharmaceutical Information |
| Drug Name |
Goserelin |
| Drug ID |
BADD_D01038 |
| Description |
Goserelin is a synthetic hormone. In men, it stops the production of the hormone testosterone, which may stimulate the growth of cancer cells. In women, goserelin decreases the production of the hormone estradiol (which may stimulate the growth of cancer cells) to levels similar to a postmenopausal state. When the medication is stopped, hormone levels return to normal. |
| Indications and Usage |
Goserelin is indicated for:
- Use in combination with flutamide for the management of locally confined carcinoma of the prostate
- Palliative treatment of advanced carcinoma of the prostate
- The management of endometriosis
- Use as an endometrial-thinning agent prior to endometrial ablation for dysfunctional uterine bleeding
- Use in the palliative treatment of advanced breast cancer in pre- and perimenopausal women |
| Marketing Status |
approved |
| ATC Code |
L02AE03 |
| DrugBank ID |
DB00014
|
| KEGG ID |
D04405
|
| MeSH ID |
D017273
|
| PubChem ID |
5311128
|
| TTD Drug ID |
D00BCG
|
| NDC Product Code |
55463-0005; 70720-951; 70720-950 |
| UNII |
0F65R8P09N
|
| Synonyms |
Goserelin | ICI-118630 | ICI 118630 | ICI118630 | Zoladex | Goserelin Acetate | Acetate, Goserelin |
|
| Chemical Information |
| Molecular Formula |
C59H84N18O14 |
| CAS Registry Number |
65807-02-5 |
| SMILES |
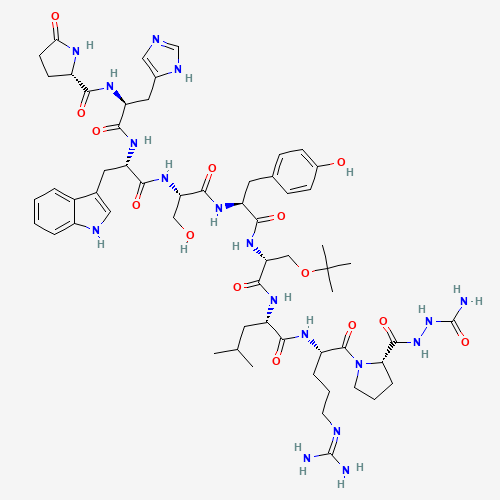
CC(C)CC(C(=O)NC(CCCN=C(N)N)C(=O)N1CCCC1C(=O)NNC(=O)N)NC(=O)C(COC(C)(C)C)NC(=O)C(
CC2=CC=C(C=C2)O)NC(=O)C(CO)NC(=O)C(CC3=CNC4=CC=CC=C43)NC(=O)C(CC5=CN=CN5)NC(=O)C
6CCC(=O)N6 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

