| Pharmaceutical Information |
| Drug Name |
Florbetapir |
| Drug ID |
BADD_D00906 |
| Description |
Florbetapir (18F) is a radiopharmaceutical compound containing the radionuclide fluorine-18 bound to the compound florbetapir, a molecule that binds with high affinity to beta amyloid plaque, a peptide that plays a key role in Alzheimer's Disease pathogenesis. Marketed as the product Amyvid, florbetapir 18F is indicated for positron emission tomography (PET) imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer's Disease (AD) and other causes of cognitive decline.
The radionucleide fluorine-18 was chosen as it has a half life of 110 minutes allowing it to accumulate sufficiently in the brain before undergoing positon emission decay. |
| Indications and Usage |
Florbetapir 18F is indicated for Positron Emission Tomography (PET) imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer's Disease (AD) and other causes of cognitive decline. |
| Marketing Status |
approved; investigational |
| ATC Code |
Not Available |
| DrugBank ID |
DB09149
|
| KEGG ID |
D09617
|
| MeSH ID |
C545186
|
| PubChem ID |
57396865
|
| TTD Drug ID |
Not Available
|
| NDC Product Code |
Not Available |
| UNII |
6867Q6IKOD
|
| Synonyms |
florbetapir | 2-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)-5-(4-methylaminostyryl)pyridine | 4-(2-(6-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)pyridin-3-yl)vinyl)-N-methylbenzamide | Amyvid | 18F-AV-45 | florbetapir F 18 | florbetapir F18 |
|
| Chemical Information |
| Molecular Formula |
C20H25FN2O3 |
| CAS Registry Number |
938435-69-9 |
| SMILES |
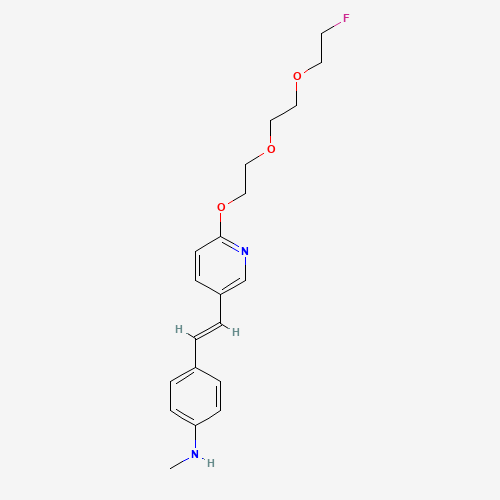
CNC1=CC=C(C=C1)C=CC2=CN=C(C=C2)OCCOCCOCCF |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

