| Pharmaceutical Information |
| Drug Name |
Ethambutol hydrochloride |
| Drug ID |
BADD_D00842 |
| Description |
Ethambutol is a bacteriostatic agent indicated alongside medications such as [isoniazid], [rifampin], and [pyrazinamide] in the treatment of pulmonary tuberculosis.[L31743] Ethambutol was first described in the literature in 1961.[A229048] It was developed out of a need for therapies active against isoniazid resistant strains of _Mycobacterium tuberculosis_.[A229048]
Ethambutol was granted FDA approval on 6 November 1967.[L31663] |
| Indications and Usage |
Ethambutol is indicated in combination with other anti-tuberculosis drugs in the treatment of pulmonary tuberculosis.[L31663] Ethambutol is commonly used in combination with [isoniazid], [rifampin], and [pyrazinamide].[L31743] |
| Marketing Status |
approved |
| ATC Code |
J04AK02 |
| DrugBank ID |
DB00330
|
| KEGG ID |
D00878
|
| MeSH ID |
D004977
|
| PubChem ID |
14051
|
| TTD Drug ID |
D08QME
|
| NDC Product Code |
42806-102; 68850-012; 68180-280; 68850-010; 70518-3662; 42806-101; 54879-002; 68084-280; 65691-0014; 68180-281; 70518-2691; 66406-0224; 50090-5140; 70518-2966; 54879-001 |
| UNII |
QE4VW5FO07
|
| Synonyms |
Ethambutol | Dexambutol | Etibi | EMB-Fatol | EMB Fatol | Etambutol Llorente | Llorente, Etambutol | Ethambutol Hydrochloride | Hydrochloride, Ethambutol | Myambutol | Miambutol | EMB-Hefa | EMB Hefa |
|
| Chemical Information |
| Molecular Formula |
C10H26Cl2N2O2 |
| CAS Registry Number |
1070-11-7 |
| SMILES |
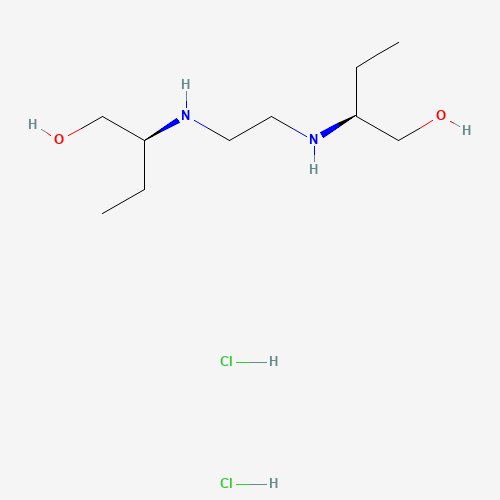
CCC(CO)NCCNC(CC)CO.Cl.Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

