| Pharmaceutical Information |
| Drug Name |
Ethacrynate sodium |
| Drug ID |
BADD_D00839 |
| Description |
A compound that inhibits symport of sodium, potassium, and chloride primarily in the ascending limb of Henle, but also in the proximal and distal tubules. This pharmacological action results in excretion of these ions, increased urinary output, and reduction in extracellular fluid. This compound has been classified as a loop or high ceiling diuretic. |
| Indications and Usage |
For the treatment of high blood pressure and edema caused by diseases like congestive heart failure, liver failure, and kidney failure. |
| Marketing Status |
approved; investigational |
| ATC Code |
C03CC01 |
| DrugBank ID |
DB00903
|
| KEGG ID |
D04079
|
| MeSH ID |
D004976
|
| PubChem ID |
23668825
|
| TTD Drug ID |
D06TNL
|
| NDC Product Code |
65571-0026; 68382-246; 69315-701; 25010-210; 63552-145; 70771-1106; 14474-042; 42413-0062; 42023-157 |
| UNII |
K41MYV7MPM
|
| Synonyms |
Ethacrynic Acid | Acid, Ethacrynic | Etacrynic Acid | Acid, Etacrynic | Ethacrinic Acid | Acid, Ethacrinic | Hydromedin | Edecrin | Ethacrynate Sodium | Sodium, Ethacrynate | Ethacrynic Acid, Sodium Salt |
|
| Chemical Information |
| Molecular Formula |
C13H11Cl2NaO4 |
| CAS Registry Number |
6500-81-8 |
| SMILES |
CCC(=C)C(=O)C1=C(C(=C(C=C1)OCC(=O)[O-])Cl)Cl.[Na+] |
| Chemical Structure |
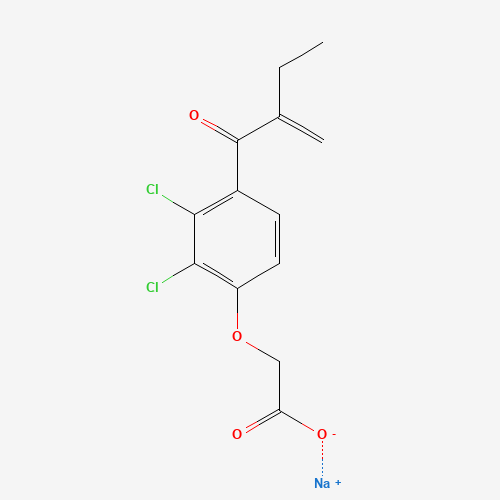
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Vision blurred | 17.17.01.010; 06.02.06.007 | - | - | | | Vomiting | 07.01.07.003 | - | - | | | Decreased appetite | 14.03.01.005; 08.01.09.028 | - | - | |
|
|
|

