| Pharmaceutical Information |
| Drug Name |
Escitalopram |
| Drug ID |
BADD_D00809 |
| Description |
Escitalopram is a selective serotonin re-uptake inhibitor (SSRI) and the S-enantiomer of racemic [citalopram].[A185420] It is used to restore serotonergic function in the treatment of depression and anxiety.[L8513,L8516,L8522] Escitalopram is approximately 150 times more potent than citalopram’s R-enantiomer and is responsible for the vast majority of citalopram’s clinical activity, with some evidence suggesting that the R-enantiomer of racemic citalopram actively dampens the activity of escitalopram rather than existing simply as an inactive enantiomer.[A39738,A185819] Amongst SSRIs, escitalopram exerts the highest degree of selectivity for the serotonin transporter (SERT) relative to other off-targets which may explain its lower rates of adverse effects as compared to other agents in this class.[A185726] Escitalopram also differentiates itself from other SSRIs via allosteric action on its target - this may be the mechanism responsible for its observed superior efficacy and faster onset compared to other SSRIs.[A185825,A185726,A185822] |
| Indications and Usage |
Escitalopram is indicated for both acute and maintenance treatment of major depressive disorder (MDD) and for the acute treatment of generalized anxiety disorder (GAD).[L8513] It is additionally indicated for symptomatic relief of obsessive-compulsive disorder (OCD) in Canada.[L8516] |
| Marketing Status |
approved |
| ATC Code |
N06AB10 |
| DrugBank ID |
DB01175
|
| KEGG ID |
D07913
|
| MeSH ID |
D000089983
|
| PubChem ID |
146570
|
| TTD Drug ID |
D08RBC
|
| NDC Product Code |
80425-0320; 50090-4915; 51655-284; 53002-1431; 67296-1200; 0456-2010; 0456-2020; 69097-848; 70518-1785; 71335-1030; 0615-8366; 42708-163; 45865-699; 55700-847; 69844-079; 72189-424; 72189-451; 16729-168; 43547-281; 43547-282; 61919-652; 63187-281; 68001-456; 68071-5248; 68788-7461; 69844-077; 70934-630; 68001-454; 68071-2035; 68645-520; 68788-7510; 69844-078; 70934-957; 71205-344; 71610-425; 71610-534; 72189-449; 16571-755; 16729-170; 50090-2196; 51655-236; 53002-2438; 60760-393; 68788-7912; 70518-2317; 70771-1145; 71335-1002; 71335-1187; 71335-1307; 16571-757; 51655-766; 54838-551; 70518-2430; 70771-1147; 50090-4363; 50090-5312; 50090-6534; 51655-116; 51655-149; 69097-847; 69097-849; 70518-1876; 71205-325; 76282-250; 76282-251; 42708-155; 50090-1930; 70518-2472; 70518-3151; 70771-1146; 82982-030; 43547-280; 50090-6190; 51655-277; 55700-818; 63187-217; 67296-1597; 68001-455; 68645-519; 0456-2005; 70518-1805; 70518-2597; 71335-1571; 72189-438; 0615-8365; 72789-194; 76282-249; 16571-756; 33342-053; 60760-170; 65162-705; 71205-782; 71335-1241; 71335-2058; 0615-8348; 16729-169; 43063-661 |
| UNII |
4O4S742ANY
|
| Synonyms |
Escitalopram | Escitalopram Oxalate | Lexapro |
|
| Chemical Information |
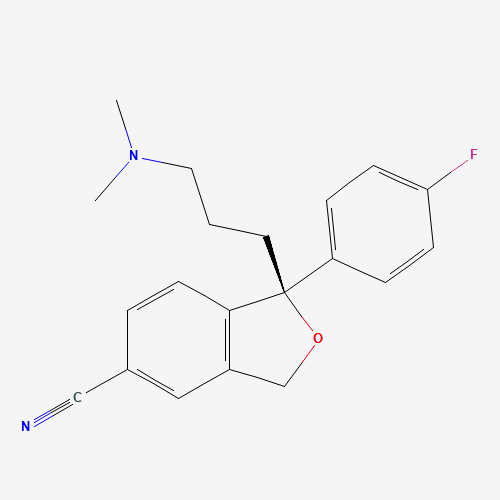
| Molecular Formula |
C20H21FN2O |
| CAS Registry Number |
128196-01-0 |
| SMILES |
CN(C)CCCC1(C2=C(CO1)C=C(C=C2)C#N)C3=CC=C(C=C3)F |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
|
|
|

