| Pharmaceutical Information |
| Drug Name |
Entecavir |
| Drug ID |
BADD_D00774 |
| Description |
Entecavir is an oral antiviral drug used in the treatment of hepatitis B infection. It is marketed under the trade name Baraclude (BMS).
Entecavir is a guanine analogue that inhibits all three steps in the viral replication process, and the manufacturer claims that it is more efficacious than previous agents used to treat hepatitis B (lamivudine and adefovir). It was approved by the U.S. Food and Drug Administration (FDA) in March 2005. |
| Indications and Usage |
For the treatment of chronic hepatitis B virus infection in adults with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease. |
| Marketing Status |
approved; investigational |
| ATC Code |
J05AF10 |
| DrugBank ID |
DB00442
|
| KEGG ID |
D04008; D07896
|
| MeSH ID |
C413685
|
| PubChem ID |
135398508
|
| TTD Drug ID |
D0KR2J
|
| NDC Product Code |
63285-887; 60687-216; 65862-841; 17381-031; 71921-195; 42806-659; 43547-436; 43547-437; 68382-920; 76397-001; 31722-833; 70771-1020; 68382-921; 65862-798; 71921-194; 16729-388; 42291-261; 63126-102; 16714-717; 50771-014; 63126-101; 70771-1019; 0003-1611; 16729-389; 42291-262; 69097-426; 13612-0020; 0003-1612; 0003-1614; 63285-888; 31722-834; 65162-446; 69097-425; 51991-895; 65162-449; 65862-842; 53104-7692; 65977-0086; 66406-0331; 16714-718; 42806-658; 50268-289; 50771-013; 51991-896 |
| UNII |
5968Y6H45M
|
| Synonyms |
entecavir | Baraclude |
|
| Chemical Information |
| Molecular Formula |
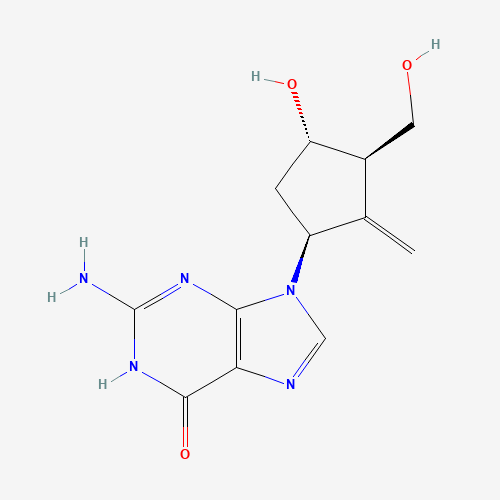
C12H15N5O3 |
| CAS Registry Number |
142217-69-4 |
| SMILES |
C=C1C(CC(C1CO)O)N2C=NC3=C2N=C(NC3=O)N |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

