| Pharmaceutical Information |
| Drug Name |
Edoxaban tosylate |
| Drug ID |
BADD_D00746 |
| Description |
Edoxaban is a member of the Novel Oral Anti-Coagulants (NOACs) class of drugs, and is a rapidly acting, oral, selective factor Xa inhibitor. By inhibiting factor Xa, a key protein in the coagulation cascade, edoxaban prevents the stepwise amplification of protein factors needed to form blood clots. It is indicated to reduce the risk of stroke and systemic embolism (SE) in patients with nonvalvular atrial fibrillation (NVAF) and for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) following 5-10 days of initial therapy with a parenteral anticoagulant. Traditionally, warfarin, a vitamin K antagonist, was used for stroke prevention in these individuals but effective use of this drug is limited by it's delayed onset, narrow therapeutic window, need for regular monitoring and INR testing, and numerous drug-drug and drug-food interactions. This has prompted enthusiasm for newer agents such as dabigatran, apixaban, and rivaroxaban for effective clot prevention. In addition to once daily dosing, the benefits over warfarin also include significant reductions in hemorrhagic stroke and GI bleeding, and improved compliance, which is beneficial as many patients will be on lifelong therapy. |
| Indications and Usage |
Edoxaban is indicated for reducing the risk of stroke and systemic embolism (SE) in patients with nonvalvular atrial fibrillation (NVAF). However, it should not be used in patients with creatinine clearance (CrCL) > 95 mL/min because of increased risk of ischemic stroke compared to warfarin at the highest dose studied (60 mg). It is also indicated for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) following 5-10 days of initial therapy with a parenteral anticoagulant. |
| Marketing Status |
approved |
| ATC Code |
B01AF03 |
| DrugBank ID |
DB09075
|
| KEGG ID |
D11568
|
| MeSH ID |
C552171
|
| PubChem ID |
44610678
|
| TTD Drug ID |
D0SL8X
|
| NDC Product Code |
65597-201; 65597-202; 65597-203; 66003-202; 66003-203; 66003-201 |
| UNII |
32W99UE810
|
| Synonyms |
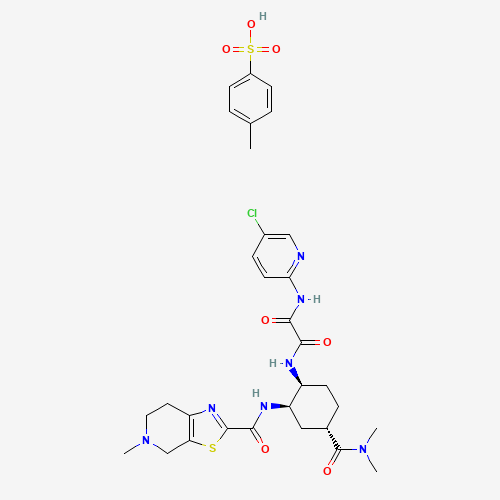
edoxaban | N-(5-chloropyridin-2-yl)-N'-((1S,2R,4S)-4-(N,N-dimethylcarbamoyl)-2-(5-methyl-4,5,6,7- tetrahydro(1,3)thiazolo(5,4-c)pyridine-2-carboxamido)cyclohexyl)oxamide | N-(5-chloropyridin-2-yl)-N'-((1S,2R,4S)-4-(N,N-dimethylcarbamoyl)-2-(5-methyl-4,5,6,7-tetrahydrothiazolo(5,4-c)pyridine-2-carboxamido)cyclohexyl)ethanediamide p-toluenesulfonate monohydrate | Savaysa | DU-176 | DU-176b | edoxaban tosylate |
|
| Chemical Information |
| Molecular Formula |
C31H38ClN7O7S2 |
| CAS Registry Number |
480449-71-6 |
| SMILES |
CC1=CC=C(C=C1)S(=O)(=O)O.CN1CCC2=C(C1)SC(=N2)C(=O)NC3CC(CCC3NC(=O)C(=O)NC4=NC=C(
C=C4)Cl)C(=O)N(C)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

