| Pharmaceutical Information |
| Drug Name |
Edetate disodium |
| Drug ID |
BADD_D00744 |
| Description |
A chelating agent (chelating agents) that sequesters a variety of polyvalent cations. It is used in pharmaceutical manufacturing and as a food additive. |
| Indications and Usage |
For the reduction of blood levels and depot stores of lead in lead poisoning (acute and chronic) and lead encephalopathy, in both pediatric populations and adults. |
| Marketing Status |
approved; vet_approved |
| ATC Code |
V03AB03 |
| DrugBank ID |
DB00974
|
| KEGG ID |
D01802
|
| MeSH ID |
D004492
|
| PubChem ID |
8759
|
| TTD Drug ID |
D08RIB
|
| NDC Product Code |
62675-2751 |
| UNII |
7FLD91C86K
|
| Synonyms |
Edetic Acid | Acid, Edetic | EDTA | Ethylenedinitrilotetraacetic Acid | Acid, Ethylenedinitrilotetraacetic | N,N'-1,2-Ethanediylbis(N-(carboxymethyl)glycine) | Edathamil | Ethylenediaminetetraacetic Acid | Acid, Ethylenediaminetetraacetic | Versene | Chelaton 3 | Copper EDTA | EDTA, Copper | Coprin | Dicobalt EDTA | EDTA, Dicobalt | Disodium Calcitetracemate | Calcitetracemate, Disodium | Distannous EDTA | EDTA, Distannous | Edetate Disodium Calcium | Calcium Disodium Edetate | Edetate, Calcium Disodium | Edetates | Edetic Acid, Calcium Salt | Edetic Acid, Calcium, Sodium Salt | Edetic Acid, Chromium Salt | Chromium EDTA | EDTA, Chromium | Edetic Acid, Dipotassium Salt | Edetic Acid, Disodium Salt | Disodium Ethylene Dinitrilotetraacetate | Dinitrilotetraacetate, Disodium Ethylene | Ethylene Dinitrilotetraacetate, Disodium | Disodium EDTA | EDTA, Disodium | Edetic Acid, Disodium Salt, Dihydrate | Edetic Acid, Disodium, Monopotassium Salt | Edetic Acid, Magnesium Salt | Edetic Acid, Monopotassium Salt | Edetic Acid, Monosodium Salt | Edetic Acid, Potassium Salt | Potassium EDTA | EDTA, Potassium | Edetic Acid, Sodium Salt | Gallium EDTA | EDTA, Gallium | Magnesium Disodium EDTA | EDTA, Magnesium Disodium | Edetic Acid, Disodium, Magnesium Salt | Stannous EDTA | EDTA, Stannous | Tetracemate | Ethylene Dinitrilotetraacetate | Dinitrilotetraacetate, Ethylene | Versenate | Calcium Disodium Versenate | Disodium Versenate, Calcium | Versenate, Calcium Disodium | Calcium Tetacine | Tetacine, Calcium |
|
| Chemical Information |
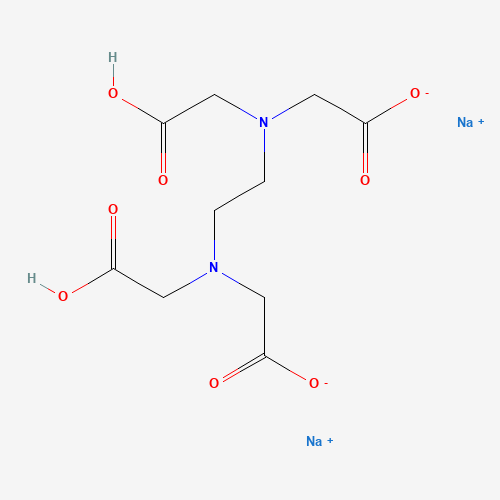
| Molecular Formula |
C10H14N2Na2O8 |
| CAS Registry Number |
139-33-3 |
| SMILES |
C(CN(CC(=O)O)CC(=O)[O-])N(CC(=O)O)CC(=O)[O-].[Na+].[Na+] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

