| Pharmaceutical Information |
| Drug Name |
Dicyclomine |
| Drug ID |
BADD_D00659 |
| Description |
Dicyclomine is a muscarinic M1, M3, and M2 receptor antagonist as well as a non-competitive inhibitor of histamine and bradykinin used to treat spasms of the intestines seen in functional bowel disorder and irritable bowel syndrome.[A6556,A182555,A234659,L7967] Though it is commonly prescribed, its recommendation may have been based on a small amount of evidence and so its prescription is becoming less favourable.[L7982]
Dicyclomine was granted FDA approval on 11 May 1950.[L7967] |
| Indications and Usage |
Dicyclomine is indicated for the treatment of functional bowel disorder and irritable bowel syndrome.[L7967] |
| Marketing Status |
approved |
| ATC Code |
A03AA07 |
| DrugBank ID |
DB00804
|
| KEGG ID |
D07820
|
| MeSH ID |
D004025
|
| PubChem ID |
3042
|
| TTD Drug ID |
D07XJM
|
| NDC Product Code |
Not Available |
| UNII |
4KV4X8IF6V
|
| Synonyms |
Dicyclomine | Dicycloverin | Dicyclomine Hydrochloride | Hydrochloride, Dicyclomine | Merbentyl | Bentylol | Spascol | Di-Spaz | Di Spaz | Dibent | Diclomin | Lomine | OR-Tyl | OR Tyl | Bentyl | Di-Cyclonex | Di Cyclonex |
|
| Chemical Information |
| Molecular Formula |
C19H35NO2 |
| CAS Registry Number |
77-19-0 |
| SMILES |
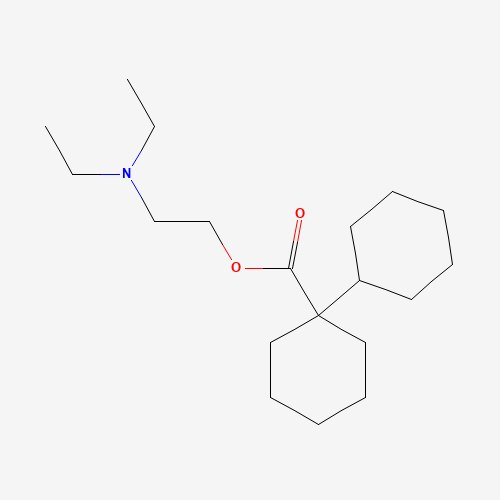
CCN(CC)CCOC(=O)C1(CCCCC1)C2CCCCC2 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

