| Pharmaceutical Information |
| Drug Name |
Dibucaine |
| Drug ID |
BADD_D00651 |
| Description |
A local anesthetic of the amide type now generally used for surface anesthesia. It is one of the most potent and toxic of the long-acting local anesthetics and its parenteral use is restricted to spinal anesthesia. (From Martindale, The Extra Pharmacopoeia, 30th ed, p1006) |
| Indications and Usage |
For production of local or regional anesthesia by infiltration techniques such as percutaneous injection and intravenous regional anesthesia by peripheral nerve block techniques such as brachial plexus and intercostal and by central neural techniques such as lumbar and caudal epidural blocks. |
| Marketing Status |
approved; vet_approved |
| ATC Code |
S01HA06; D04AB02; C05AD04; N01BB06; S02DA04 |
| DrugBank ID |
DB00527
|
| KEGG ID |
D00733
|
| MeSH ID |
D003992
|
| PubChem ID |
3025
|
| TTD Drug ID |
D0P5GE
|
| NDC Product Code |
45802-050; 0536-1211; 81266-920; 71399-2829 |
| UNII |
L6JW2TJG99
|
| Synonyms |
Dibucaine | Cinchocaine | Sovcaine | Nupercaine | Cincain | Nupercainal |
|
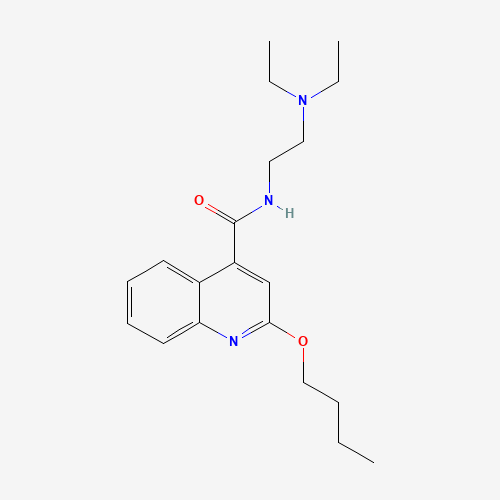
| Chemical Information |
| Molecular Formula |
C20H29N3O2 |
| CAS Registry Number |
85-79-0 |
| SMILES |
CCCCOC1=NC2=CC=CC=C2C(=C1)C(=O)NCCN(CC)CC |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Haemorrhage | 24.07.01.002 | - | - | Not Available |
|
The 1th Page
1
Total 1 Pages
|
|

