| Pharmaceutical Information |
| Drug Name |
Dabrafenib |
| Drug ID |
BADD_D00566 |
| Description |
Dabrafenib mesylate (Tafinlar) is a reversible ATP-competitive kinase inhibitor and targets the MAPK pathway. It was approved on May 29, 2013 for the treatment of melanoma [L2718].
In May 2018, Tafinlar (dabrafenib) and Mekinist ([DB08911]) in combination have been approved to treat anaplastic thyroid cancer caused by an abnormal BRAF V600E gene [L2714]. |
| Indications and Usage |
Tafinlar is a kinase inhibitor that was initially indicated as a single agent for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E mutation as detected by an FDA-approved test [FDA label].
Tafinlar in combination with [DB08911] is indicated for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations as detected by an FDA-approved test. The use in combination is based on the demonstration of durable response rate. Improvement in disease-related symptoms or overall survival has not been demonstrated for Tafinlar in combination with trametinib [FDA label].
In May 2018, Tafinlar (dabrafenib) and Mekinist ([DB08911]) have been approved in combination to treat anaplastic thyroid cancer caused by an abnormal BRAF V600E gene [L2712]. |
| Marketing Status |
approved; investigational |
| ATC Code |
L01EC02 |
| DrugBank ID |
DB08912
|
| KEGG ID |
D10064
|
| MeSH ID |
C561627
|
| PubChem ID |
44462760
|
| TTD Drug ID |
D05ROI
|
| NDC Product Code |
0078-0681; 0078-0682; 0078-1154; 52482-007 |
| UNII |
QGP4HA4G1B
|
| Synonyms |
dabrafenib | GSK 2118436 | GSK2118436 | GSK-2118436 |
|
| Chemical Information |
| Molecular Formula |
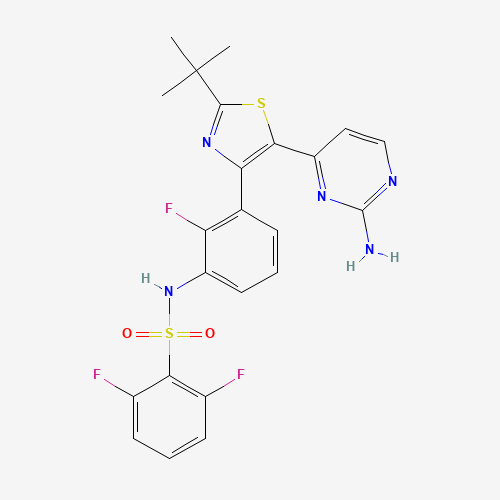
C23H20F3N5O2S2 |
| CAS Registry Number |
1195765-45-7 |
| SMILES |
CC(C)(C)C1=NC(=C(S1)C2=NC(=NC=C2)N)C3=C(C(=CC=C3)NS(=O)(=O)C4=C(C=CC=C4F)F)F |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

