| Pharmaceutical Information |
| Drug Name |
Dabigatran etexilate |
| Drug ID |
BADD_D00564 |
| Description |
Dabigatran etexilate is an oral prodrug that is hydrolyzed to the competitive and reversible direct thrombin inhibitor [dabigatran].[A177463, A6970, L34675, L34680] Dabigatran etexilate may be used to decrease the risk of venous thromboembolic events in patients in whom anticoagulation therapy is indicated.[A177463] In contrast to warfarin, because its anticoagulant effects are predictable, lab monitoring is not necessary.[A177463] Dabigatran etexilate was approved by the FDA in 2010.[L6022] |
| Indications and Usage |
Dabigatran etexilate is available in both oral pellet and capsule form. Dabigatran etexilate pellets are indicated for the treatment of venous thromboembolic events (VTE) in pediatric patients between three months and 12 years of age who have been treated with a parenteral anticoagulant for at least 5 days. They are also indicated in the same age group to reduce the risk of recurrence of VTE in patients who have been previously treated.[L34675]
In capsule form, dabigatran etexilate is indicated in adults to reduce the risk of stroke and systemic embolism associated with non-valvular atrial fibrillation and for the treatment of deep venous thrombosis (DVT) and pulmonary embolism (PE) in patients who have been treated with a parenteral anticoagulant for 5-10 days. It is also indicated in adults to reduce the risk of recurrence of DVT and PE in patients who have been previously treated and for the prophylaxis of DVT and PE in patients who have undergone hip replacement surgery. Lastly, it is indicated in pediatric patients between eight and 18 years of age for the treatment of venous thromboembolic events (VTE) in patients who have been treated with a parenteral anticoagulant for at least 5 days and to reduce the risk of recurrence of VTE in patients who have been previously treated.[L34680] |
| Marketing Status |
approved |
| ATC Code |
B01AE07 |
| DrugBank ID |
DB06695
|
| KEGG ID |
D07144
|
| MeSH ID |
D000069604
|
| PubChem ID |
135565674
|
| TTD Drug ID |
D0KI5R
|
| NDC Product Code |
67877-475; 67877-474; 31722-621; 60687-744; 60687-752; 0597-0430; 0597-0445; 31722-622; 0597-0425; 0597-0435; 0597-0440; 0597-0450 |
| UNII |
2E18WX195X
|
| Synonyms |
Dabigatran | N-((2-(((4-(aminoiminomethyl)phenyl)amino)methyl)-1-methyl-1H-benzimidazol-5-yl)carbonyl)-N-2-pyridinyl-beta-alanine | BIBR 1048 | Pradaxa | Dabigatran Etexilate | Etexilate, Dabigatran | Dabigatran Etexilate Mesylate | Etexilate Mesylate, Dabigatran | Mesylate, Dabigatran Etexilate |
|
| Chemical Information |
| Molecular Formula |
C34H41N7O5 |
| CAS Registry Number |
211915-06-9 |
| SMILES |
CCCCCCOC(=O)NC(=N)C1=CC=C(C=C1)NCC2=NC3=C(N2C)C=CC(=C3)C(=O)N(CCC(=O)OCC)C4=CC=C
C=N4 |
| Chemical Structure |
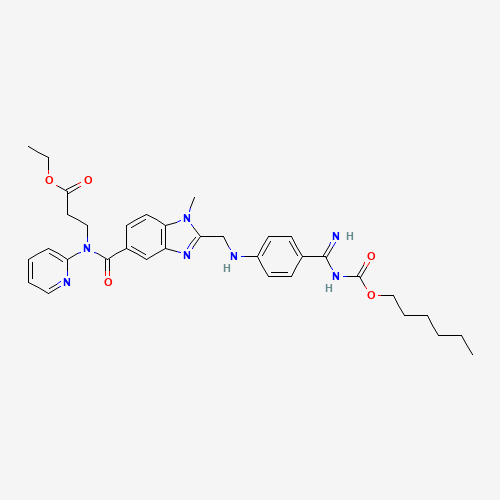
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
|
|
|

