| Pharmaceutical Information |
| Drug Name |
Cyclosporine |
| Drug ID |
BADD_D00551 |
| Description |
Cyclosporine is a calcineurin inhibitor known for its immunomodulatory properties that prevent organ transplant rejection and treat various inflammatory and autoimmune conditions. It is isolated from the fungus _Beauveria nivea_.[A174049] Initially manufactured by Sandoz and approved for use by the FDA in 1983, cyclosporine is now available in various products by Novartis (previously known as Sandoz).[L11097,L3734,L11118] |
| Indications and Usage |
Cyclosporine is approved for a variety of conditions. Firstly, it is approved for the prophylaxis of organ rejection in allogeneic kidney, liver, and heart transplants. It is also used to prevent bone marrow transplant rejection. For the above indications, cyclosporine can be used in conjunction with azathioprine and corticosteroids. Finally, cyclosporine can be used in patients who have chronic transplant rejection and have received previous immunosuppressive therapy[L3002] and to prevent or treat graft-versus-host disease (GVHD).[L11097]
Secondly, cyclosporine is used for the treatment of patients with severe active rheumatoid arthritis (RA) when they no longer respond to methotrexate alone.[L3734] It can be used for the treatment of adult non-immunocompromised patients with severe, recalcitrant, plaque psoriasis that have failed to respond to at least one systemic therapy or when systemic therapies are not tolerated or contraindicated.[L3734] The ophthalmic solution of cyclosporine is indicated to increase tear production in patients suffering from keratoconjunctivitis sicca.[L11097] In addition, cyclosporine is approved for the treatment of steroid dependent and steroid-resistant nephrotic syndrome due to glomerular diseases which may include minimal change nephropathy, focal and segmental glomerulosclerosis or membranous glomerulonephritis.[L11097]
A cyclosporine ophthalmic emulsion is indicated in the treatment of vernal keratoconjunctivitis in adults and children.[L34694]
Off-label, cyclosporine is commonly used for the treatment of various autoimmune and inflammatory conditions such as atopic dermatitis, blistering disorders, ulcerative colitis, juvenile rheumatoid arthritis, uveitis, connective tissue diseases, as well as idiopathic thrombocytopenic purpura.[A139,A174085,A189393,A189396,A189399] |
| Marketing Status |
approved; investigational; vet_approved |
| ATC Code |
S01XA18; L04AD01 |
| DrugBank ID |
DB00091
|
| KEGG ID |
D00184
|
| MeSH ID |
D016572
|
| PubChem ID |
5280754
|
| TTD Drug ID |
D0O3YF
|
| NDC Product Code |
10888-5040; 11014-0033; 11014-0034; 11014-0041; 51927-0160; 55361-0025; 55361-0023; 23155-838; 0172-7313; 64380-127; 64380-128; 68084-921; 51927-3196; 55486-1567; 55486-1604; 55500-0009; 0093-9020; 51862-460; 60505-0134; 64380-129; 65086-001; 0574-0866; 47848-004; 51552-0663; 59573-0001; 66507-0001; 0074-3108; 0078-0241; 23155-837; 23155-839; 47335-506; 60505-0133; 0378-8760; 65897-3008; 0078-0110; 60505-4630; 60505-4632; 63629-8640; 65897-1008; 0078-0246; 0078-0274; 0093-9019; 68084-879; 11014-0040; 51927-0213; 55361-0022; 55361-0026; 68254-2501; 0078-0248; 47335-507; 60505-4631; 60505-6202; 68180-214; 10888-5041; 52928-005; 52972-0027; 55486-1602; 10702-808; 68254-0008; 0074-3109; 0074-7269; 0078-0109; 0078-0240; 0023-5301; 0074-5503; 51862-458; 55486-1603; 0093-9018; 0023-9163 |
| UNII |
83HN0GTJ6D
|
| Synonyms |
Cyclosporine | Cyclosporine A | Cyclosporin A | Ciclosporin | Cyclosporin | Neoral | Sandimmun Neoral | CyA-NOF | CyA NOF | Sandimmune | Sandimmun | CsA-Neoral | CsA Neoral | CsANeoral | OL 27-400 | OL 27 400 | OL 27400 |
|
| Chemical Information |
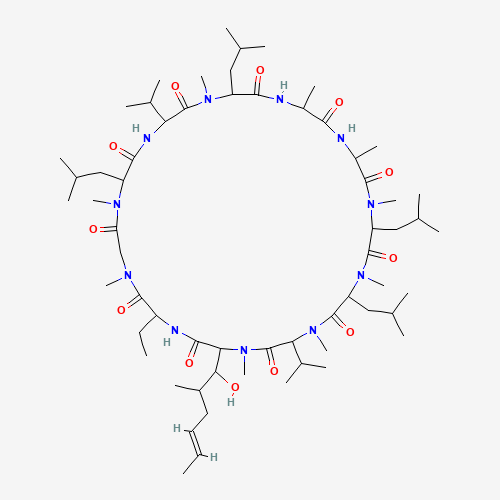
| Molecular Formula |
C62H111N11O12 |
| CAS Registry Number |
59865-13-3 |
| SMILES |
CCC1C(=O)N(CC(=O)N(C(C(=O)NC(C(=O)N(C(C(=O)NC(C(=O)NC(C(=O)N(C(C(=O)N(C(C(=O)N(C
(C(=O)N(C(C(=O)N1)C(C(C)CC=CC)O)C)C(C)C)C)CC(C)C)C)CC(C)C)C)C)C)CC(C)C)C)C(C)C)C
C(C)C)C)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
|
|
|

