| Pharmaceutical Information |
| Drug Name |
Clopidogrel |
| Drug ID |
BADD_D00504 |
| Description |
Clopidogrel is a prodrug of a platelet inhibitor used to reduce the risk of myocardial infarction and stroke.[A180508,L7213] Clopidogrel is indicated to reduce the risk of myocardial infarction for patients with non-ST elevated acute coronary syndrome (ACS), patients with ST-elevated myocardial infarction, and in recent MI, stroke, or established peripheral arterial disease,[L7213]
It has been shown to be superior to [aspirin] in reducing cardiovascular outcomes in patients with cardiovascular disease and provides additional benefit to patients with acute coronary syndromes already taking aspirin.[A180547]
Clopidogrel was granted FDA approval on 17 November 1997.[L7213] |
| Indications and Usage |
Clopidogrel is indicated to reduce the risk of myocardial infarction for patients with non-ST elevated acute coronary syndrome (ACS), patients with ST-elevated myocardial infarction, and in recent MI, stroke, or established peripheral arterial disease,[L7213] |
| Marketing Status |
approved |
| ATC Code |
B01AC04 |
| DrugBank ID |
DB00758
|
| KEGG ID |
D07729; D10823; D10824
|
| MeSH ID |
D000077144
|
| PubChem ID |
60606
|
| TTD Drug ID |
D0N0TZ
|
| NDC Product Code |
42543-714; 50090-4918; 52605-083; 16729-218; 0024-1171; 68788-8190; 72578-012; 65977-0037; 52605-082; 63187-362; 63629-4728; 70518-0400; 33342-060; 42543-713; 65162-414; 0093-7314; 16729-219; 61919-007; 67296-1840; 50090-2598; 50090-5781; 0024-1332; 61919-737; 70771-1062; 16714-052; 68071-2813; 68071-4138; 71335-0581 |
| UNII |
A74586SNO7
|
| Synonyms |
Clopidogrel | SC 25989C | SC 25990C | SR 25989 | Clopidogrel-Mepha | Clopidogrel Mepha | Clopidogrel Sandoz | Iscover | Clopidogrel Napadisilate | Clopidogrel Hydrochloride | PCR 4099 | PCR-4099 | Clopidogrel Besylate | Clopidogrel Besilate | Clopidogrel, (+)(S)-isomer | Plavix | Clopidogrel Bisulfate |
|
| Chemical Information |
| Molecular Formula |
C16H16ClNO2S |
| CAS Registry Number |
113665-84-2 |
| SMILES |
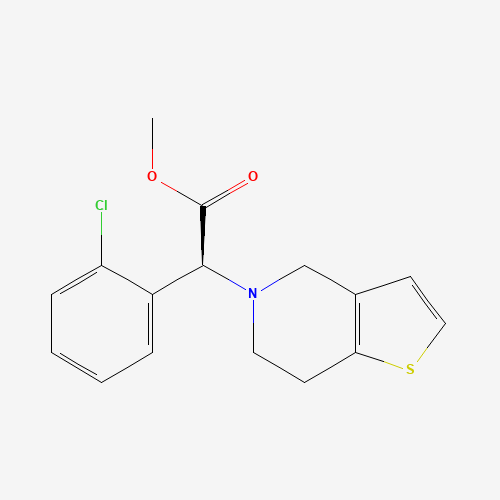
COC(=O)C(C1=CC=CC=C1Cl)N2CCC3=C(C2)C=CS3 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
|
|
|

