| Pharmaceutical Information |
| Drug Name |
Citalopram |
| Drug ID |
BADD_D00475 |
| Description |
Citalopram belongs to a class of antidepressant agents known as selective _serotonin-reuptake inhibitors_ (SSRIs) and is widely used to treat the symptoms of depression. Its chemical structure is unrelated to that of other SSRIs or of tricyclic, tetracyclic, or other prescribed antidepressants [FDA label]. Citalopram is also known as _Celexa_, and available in tablet and solution forms [FDA label]. This drug was initially approved by the FDA in 1998 [L5230]. |
| Indications and Usage |
For the treatment of depression, as indicated by the FDA label [FDA label]. Off-label indications include but are not limited to: treatment of sexual dysfunction, post-stroke behavioural changes, ethanol abuse, obsessive-compulsive disorder (OCD) in children, and diabetic neuropathy [FDA label], [A321], [A322], [A323], [A324], [A174406], [A174409], [A174412]. |
| Marketing Status |
approved |
| ATC Code |
N06AB04 |
| DrugBank ID |
DB00215
|
| KEGG ID |
D07704
|
| MeSH ID |
D015283
|
| PubChem ID |
2771
|
| TTD Drug ID |
D0Y5DO
|
| NDC Product Code |
42806-020; 71335-0541; 76282-206; 80425-0093; 37662-0340; 43353-091; 54458-980; 0054-0062; 70518-2601; 63850-3616; 0456-4040; 68071-3034; 68788-7899; 70518-2228; 76282-207; 76282-629; 43353-112; 43353-208; 50090-5172; 50090-5175; 61919-390; 0378-6231; 69097-822; 69097-824; 71610-092; 37662-0341; 37662-0342; 52427-691; 65162-053; 0378-6233; 69097-823; 60429-175; 62135-540; 65162-052; 0378-6232; 70518-2553; 70518-2617; 70518-2671; 37662-0339; 37662-0343; 50090-5170; 51655-137; 60429-173; 60429-174; 61919-389; 0456-4020; 42806-021; 54458-889; 54458-981; 65162-054; 0456-4010; 76282-628; 37662-0337; 37662-0338; 42806-019; 51655-938; 71610-412; 71610-422; 76282-208; 37662-0336; 43063-063; 51655-209 |
| UNII |
0DHU5B8D6V
|
| Synonyms |
Citalopram | Cytalopram | Citalopram Hydrobromide | Lu-10-171 | Lu10171 | Seropram | Celexa |
|
| Chemical Information |
| Molecular Formula |
C20H21FN2O |
| CAS Registry Number |
59729-33-8 |
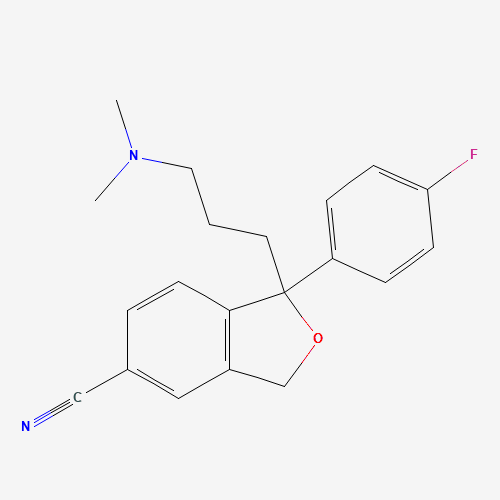
| SMILES |
CN(C)CCCC1(C2=C(CO1)C=C(C=C2)C#N)C3=CC=C(C=C3)F |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
|
|
|

