| Pharmaceutical Information |
| Drug Name |
Choline c-11 |
| Drug ID |
BADD_D00454 |
| Description |
Choline C 11 Injection is a radioactive diagnostic agent for positron emission tomography (PET) imaging of pat ients with suspected prostate cancer recurrence and non-informative bone scintigraphy, computerized tomography (CT) or magnetic resonance imaging. |
| Indications and Usage |
Choline C 11 Injection is indicated for positron emission tomography (PET) imaging of patients with suspected prostate cancer recurrence and non-informative bone scintigraphy, computerized tomography (CT) or magnetic resonance imaging (MRI). In these patients, 11 C-choline PET imaging may help identify potential sites of prostate cancer recurrence for subsequent histologic confirmation. Suspected prostate recurrence is based upon elevated blood prostate specific antigen (PSA) levels following initial therapy. In clinical studies, imag es w ere produced with PET/CT coregistration. |
| Marketing Status |
approved |
| ATC Code |
Not Available |
| DrugBank ID |
DB09563
|
| KEGG ID |
D07690
|
| MeSH ID |
D002794
|
| PubChem ID |
449688
|
| TTD Drug ID |
D0C1QZ
|
| NDC Product Code |
52670-556 |
| UNII |
M4AS4XGD4Q
|
| Synonyms |
Choline | 2-Hydroxy-N,N,N-trimethylethanaminium | Choline Citrate | Citrate, Choline | Choline O-Sulfate | Choline O Sulfate | O-Sulfate, Choline | Choline Hydroxide | Hydroxide, Choline | Bursine | Vidine | Fagine | Choline Bitartrate | Bitartrate, Choline | Choline Chloride | Chloride, Choline |
|
| Chemical Information |
| Molecular Formula |
C5H14NO+ |
| CAS Registry Number |
94793-58-5 |
| SMILES |
C[N+](C)(C)CCO |
| Chemical Structure |
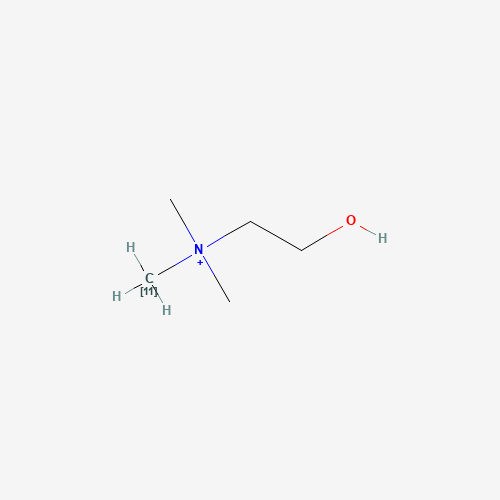
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Injection site reaction | 12.07.03.015; 08.02.03.014 | - | - | |
|
The 1th Page
1
Total 1 Pages
|
|

