| Pharmaceutical Information |
| Drug Name |
Cholecalciferol |
| Drug ID |
BADD_D00450 |
| Description |
Vitamin D, in general, is a secosteroid generated in the skin when 7-dehydrocholesterol located there interacts with ultraviolet irradiation - like that commonly found in sunlight [L5689]. Both the endogenous form of vitamin D (that results from 7-dehydrocholesterol transformation), vitamin D3 (cholecalciferol), and the plant-derived form, vitamin D2 (ergocalciferol), are considered the main forms of vitamin d and are found in various types of food for daily intake [L5689]. Structurally, ergocalciferol differs from cholecalciferol in that it possesses a double bond between C22 and C23 and has an additional methyl group at C24 [L5689]. Finally, ergocalciferol is pharmacologically less potent than cholecalciferol, which makes vitamin D3 the preferred agent for medical use [L5689].
Appropriate levels of vitamin D must be upheld in the body in order to maintain calcium and phosphorus levels in a healthy physiologic range to sustain a variety of metabolic functions, transcription regulation, and bone metabolism [A223, L5689, L1782, L5771, F4027, F4042, F4048]. However, studies are also ongoing to determine whether or not cholecalciferol may also play certain roles in cancer, autoimmune disorders, cardiovascular disease, and other medical conditions that may be associated with vitamin D deficiency [L5689]. |
| Indications and Usage |
Cholecalciferol use is indicated for the treatment of specific medical conditions like refractory rickets (or vitamin D resistant rickets), hypoparathyroidism, and familial hypophosphatemia [F4027, F4042].
Concurrently, as one of the most commonly utilized forms of vitamin D, cholecalciferol is also very frequently used as a supplement in individuals to maintain sufficient vitamin d levels in the body or to treat vitamin D deficiency, as well as various medical conditions that can be associated directly or indirectly with vitamin d insufficiency like osteoporosis and chronic kidney disease, among others [A176041, A176044, F4051]. |
| Marketing Status |
approved; nutraceutical |
| ATC Code |
Not Available |
| DrugBank ID |
DB00169
|
| KEGG ID |
D00188
|
| MeSH ID |
D002762
|
| PubChem ID |
5280795
|
| TTD Drug ID |
D0K5WS
|
| NDC Product Code |
63238-4220; 63238-4210; 63238-4200; 63238-4230 |
| UNII |
1C6V77QF41
|
| Synonyms |
Cholecalciferol | Calciol | (3 beta,5Z,7E)-9,10-Secocholesta-5,7,10(19)-trien-3-ol | Vitamin D 3 | Vitamin D3 | Cholecalciferols |
|
| Chemical Information |
| Molecular Formula |
C27H44O |
| CAS Registry Number |
67-97-0 |
| SMILES |
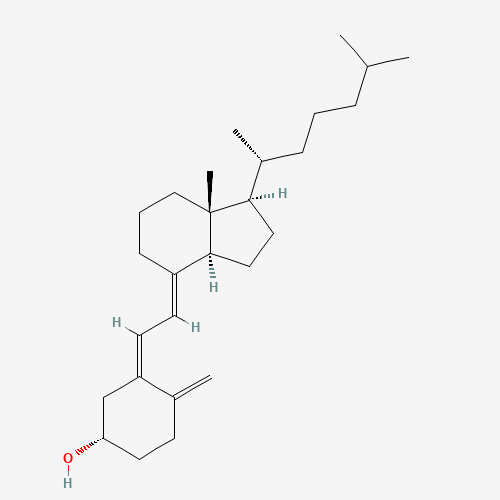
CC(C)CCCC(C)C1CCC2C1(CCCC2=CC=C3CC(CCC3=C)O)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Nausea | 07.01.07.001 | - | - | |
|
The 1th Page
1
Total 1 Pages
|
|

