| Pharmaceutical Information |
| Drug Name |
Chloroquine |
| Drug ID |
BADD_D00440 |
| Description |
Chloroquine is an aminoquinolone derivative first developed in the 1940s for the treatment of malaria.[A191655] It was the drug of choice to treat malaria until the development of newer antimalarials such as [pyrimethamine], [artemisinin], and [mefloquine].[A191787] Chloroquine and its derivative [hydroxychloroquine] have since been repurposed for the treatment of a number of other conditions including HIV, systemic lupus erythematosus, and rheumatoid arthritis.[A192432]
**The FDA emergency use authorization for [hydroxychloroquine] and chloroquine in the treatment of COVID-19 was revoked on 15 June 2020.[L14312]**
Chloroquine was granted FDA Approval on 31 October 1949.[L12054] |
| Indications and Usage |
Chloroquine is indicated to treat infections of _P. vivax_, _P. malariae_, _P. ovale_, and susceptible strains of _P. falciparum_.[L12051] It is also used to treat extraintestinal amebiasis.[L12051]
Chloroquine is also used off label for the treatment of rheumatic diseases,[A191655] as well as treatment and prophylaxis of Zika virus.[A191649,A191652] Chloroquine is currently undergoing clinical trials for the treatment of COVID-19.[A191631] |
| Marketing Status |
approved; investigational; vet_approved |
| ATC Code |
P01BA01 |
| DrugBank ID |
DB00608
|
| KEGG ID |
D02366
|
| MeSH ID |
D002738
|
| PubChem ID |
2719
|
| TTD Drug ID |
D09EGZ
|
| NDC Product Code |
Not Available |
| UNII |
886U3H6UFF
|
| Synonyms |
Chloroquine | Chlorochin | Chingamin | Khingamin | Nivaquine | Chloroquine Sulfate | Sulfate, Chloroquine | Chloroquine Sulphate | Sulphate, Chloroquine | Aralen | Arequin | Arechine |
|
| Chemical Information |
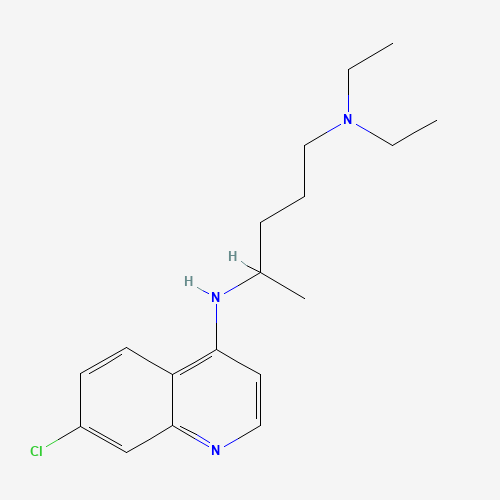
| Molecular Formula |
C18H26ClN3 |
| CAS Registry Number |
54-05-7 |
| SMILES |
CCN(CC)CCCC(C)NC1=C2C=CC(=CC2=NC=C1)Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Cyanosis central | 02.11.04.005; 22.02.02.012 | 0.000142% | | Not Available | | Pupil fixed | 17.02.11.008; 06.05.03.012 | 0.000142% | | Not Available | | Dilated cardiomyopathy | 02.04.01.017 | 0.000213% | | Not Available | | Discordant twin | 18.03.01.005 | 0.000142% | | Not Available |
|
|
|

