| Pharmaceutical Information |
| Drug Name |
Chlorhexidine diacetate |
| Drug ID |
BADD_D00435 |
| Description |
Chlorhexidine is a broad-spectrum antimicrobial biguanide used as a topical antiseptic and in dental practice for the treatment of inflammatory dental conditions caused by microorganisms.[L11512] It is one of the most common skin and mucous membrane antiseptic agents in use today.[A190417] The molecule itself is a cationic bis-guanide consisting of two 4-chlorophenyl rings and two biguanide groups joined by a central hexamethylene chain.[A190453] Topical chlorhexidine for disinfection, as well as oral rinses for dental use, carries activity against a broad range of pathogens including bacteria, yeasts, and viruses.[L11518,L11512,A190453]
Chlorhexidine was developed in the UK by Imperial Chemical Industries in the early 1950s[A190474] and was introduced to the US in the 1970s.[L11572] |
| Indications and Usage |
Chlorhexidine is available over-the-counter in various formulations (e.g. solution, sponge, cloth, swab) as a topical antiseptic to sanitize prior to surgeries and/or medical procedures.[L11518,L11521,L11527,L11533] Dental formulations, available by prescription only, include an oral rinse indicated for the treatment of gingivitis[L11512] and a slow-release "chip" which is inserted into periodontal pockets and is indicated for the reduction of pocket depth in adult patients with periodontitis as an adjunct therapy to dental scaling and root planing procedures.[L11536] |
| Marketing Status |
approved; vet_approved |
| ATC Code |
B05CA02; A01AB03; R02AA05; D09AA12; S02AA09; S01AX09; D08AC02; S03AA04 |
| DrugBank ID |
DB00878
|
| KEGG ID |
D07669
|
| MeSH ID |
D002710
|
| PubChem ID |
9562059
|
| TTD Drug ID |
D0V4GY
|
| NDC Product Code |
76107-135; 53296-0010 |
| UNII |
R4KO0DY52L
|
| Synonyms |
Chlorhexidine | Chlorhexidine Hydrochloride | Hydrochloride, Chlorhexidine | Tubulicid | Novalsan | Sebidin A | Chlorhexidine Acetate | Acetate, Chlorhexidine | MK-412A | MK 412A | MK412A |
|
| Chemical Information |
| Molecular Formula |
C26H38Cl2N10O4 |
| CAS Registry Number |
56-95-1 |
| SMILES |
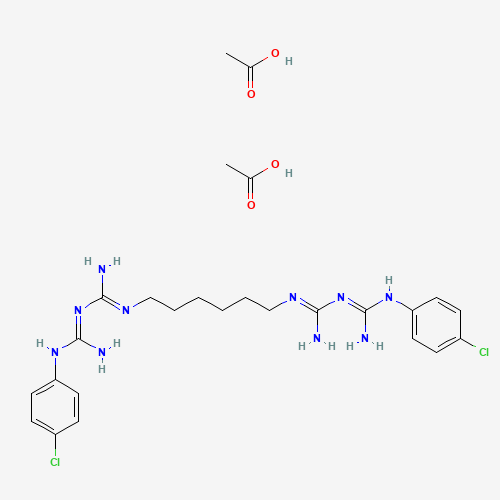
CC(=O)O.CC(=O)O.C1=CC(=CC=C1NC(=NC(=NCCCCCCN=C(N)N=C(N)NC2=CC=C(C=C2)Cl)N)N)Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Skin irritation | 23.03.04.009 | - | - | Not Available |
|
The 1th Page
1
Total 1 Pages
|
|

