| Pharmaceutical Information |
| Drug Name |
Ceftazidime |
| Drug ID |
BADD_D00399 |
| Description |
Bacteria possess a cell wall comprising a glycopeptide polymer commonly known as peptidoglycan, which is synthesized and remodelled through the action of a family of enzymes known as "penicillin-binding proteins" (PBPs).[A232920] β-lactam antibiotics, including cephalosporins, are PBP inhibitors that, through inhibition of essential PBPs, result in impaired cell wall homeostasis, loss of cell integrity, and ultimately bacterial cell death.[A232920, A232925, A232930] Ceftazidime is a third-generation cephalosporin with broad-spectrum antibacterial activity, including against some treatment-resistant bacteria such as _Pseudomonas aeruginosa_.[L32935]
Ceftazidime was approved by the FDA on July 19, 1985, and is currently available either alone or in combination with the non-β-lactam β-lactamase inhibitor [avibactam] to treat a variety of bacterial infections.[L32935, L32940] |
| Indications and Usage |
Ceftazidime is indicated for the treatment of lower respiratory tract infections, skin and skin structure infections, urinary tract infections, bacterial septicemia, bone and joint infections, gynecologic infections, intra-abdominal infections (including peritonitis), and central nervous system infections (including meningitis) caused by susceptible bacteria.[L32935]
Ceftazidime is indicated in combination with [avibactam] to treat infections caused by susceptible Gram-negative organisms, including complicated intra-abdominal infections (cIAI), in conjunction with [metronidazole], and complicated urinary tract infections (cUTI), including pyelonephritis, in patients aged three months and older. This combination is also indicated to treat hospital-acquired and ventilator-associated bacterial pneumonia (HABP/VABP) in patients aged 18 years and older.[L32940]
In all cases, to mitigate the risk of bacterial resistance and preserve clinical efficacy, ceftazidime should only be used for infections that are confirmed or strongly suspected to be caused by susceptible bacterial strains.[L32935, L32940] |
| Marketing Status |
approved |
| ATC Code |
J01DD02 |
| DrugBank ID |
DB00438
|
| KEGG ID |
D00921; D07654
|
| MeSH ID |
D002442
|
| PubChem ID |
5481173
|
| TTD Drug ID |
D0PH5Z
|
| NDC Product Code |
0264-3145; 25021-127; 0409-5082; 10402-013; 0409-5084; 0409-5086; 0409-5092; 82608-010; 0409-5093; 25021-128; 44567-235; 0264-3143; 52946-0924; 44567-237; 25021-129; 44567-236 |
| UNII |
9M416Z9QNR
|
| Synonyms |
Ceftazidime | Fortaz | Fortum | Ceftazidime Pentahydrate | LY-139381 | LY 139381 | LY139381 | Tazidime | Ceftazidime Anhydrous | Pyridinium, 1-((7-(((2-amino-4-thiazolyl)((1-carboxy-1-methylethoxy)imino)acetyl)amino)-2-carboxy-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)-, inner salt, pentahydrate, (6R-(6alpha,7beta(Z)))- | GR-20263 | GR 20263 | GR20263 |
|
| Chemical Information |
| Molecular Formula |
C22H22N6O7S2 |
| CAS Registry Number |
72558-82-8 |
| SMILES |
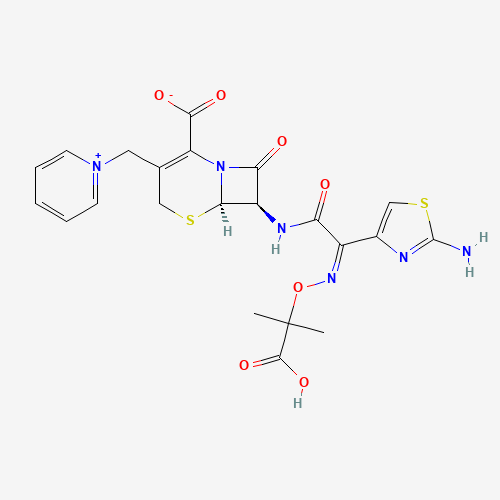
CC(C)(C(=O)O)ON=C(C1=CSC(=N1)N)C(=O)NC2C3N(C2=O)C(=C(CS3)C[N+]4=CC=CC=C4)C(=O)[O
-] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

