| Pharmaceutical Information |
| Drug Name |
Carteolol hydrochloride |
| Drug ID |
BADD_D00373 |
| Description |
A beta-adrenergic antagonist used as an anti-arrhythmia agent, an anti-angina agent, an antihypertensive agent, and an antiglaucoma agent. |
| Indications and Usage |
For the treatment of intraocular hypertension and chronic open-angle glaucoma |
| Marketing Status |
Prescription; Discontinued |
| ATC Code |
C07AA15; S01ED05 |
| DrugBank ID |
DB00521
|
| KEGG ID |
D00599
|
| MeSH ID |
D002354
|
| PubChem ID |
40127
|
| TTD Drug ID |
D03GCJ
|
| NDC Product Code |
43898-0104; 61314-238 |
| Synonyms |
Carteolol | OPC-1085 | OPC 1085 | OPC1085 | Carteolol Hydrochloride | Hydrochloride, Carteolol | Carteolol Monohydrochloride | Monohydrochloride, Carteolol |
|
| Chemical Information |
| Molecular Formula |
C16H25ClN2O3 |
| CAS Registry Number |
51781-21-6 |
| SMILES |
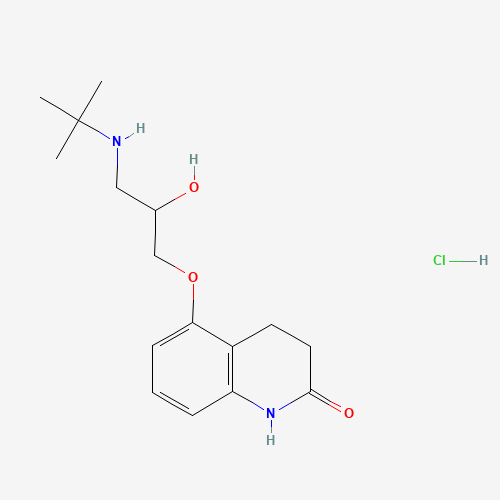
CC(C)(C)NCC(COC1=CC=CC2=C1CCC(=O)N2)O.Cl |
| Chemical Structure |
|
|
| ADR Related Proteins Induced by Drug |
| ADR Term |
Protein Name |
UniProt AC |
TTD Target ID |
PMID |
| Not Available | Not Available | Not Available | Not Available | Not Available |
|
| ADRs Induced by Drug |
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Conjunctival hyperaemia | 06.04.01.004 | - | - | Not Available |
|
|
|

