| Pharmaceutical Information |
| Drug Name |
Capsaicin |
| Drug ID |
BADD_D00353 |
| Description |
Capsaicin is most often used as a topical analgesic and exists in many formulations of cream, liquid, and patch preparations of various strengths; however, it may also be found in some dietary supplements. Capsaicin is a naturally-occurring botanical irritant in chili peppers, synthetically derived for pharmaceutical formulations. The most recent capsaicin FDA approval was Qutenza, an 8% capsaicin patch dermal-delivery system, indicated for neuropathic pain associated with post-herpetic neuralgia. |
| Indications and Usage |
The capsaicin 8% patch is indicated in the treatment of neuropathic pain associated with post-herpetic neuralgia. There are multiple topical capsaicin formulations available, including creams and solutions, indicated for temporary analgesia in muscle and join pain as well as neuropathic pain. |
| Marketing Status |
approved |
| ATC Code |
M02AB01; N01BX04 |
| DrugBank ID |
DB06774
|
| KEGG ID |
D00250
|
| MeSH ID |
D002211
|
| PubChem ID |
1548943
|
| TTD Drug ID |
D0U5CE
|
| NDC Product Code |
73102-046; 83158-387; 24286-1559; 45865-773; 49671-001; 49671-002; 59779-381; 68016-067; 69420-6025; 70656-742; 71480-831; 72490-018; 72490-023; 72490-026; 72512-929; 79643-002; 66499-0036; 11822-7448; 50268-195; 50488-1075; 50488-2025; 59088-310; 0363-9902; 69420-7025; 0536-2525; 72490-024; 72512-928; 75249-006; 51686-0002; 41167-7514; 49873-608; 51672-5308; 59316-996; 63187-660; 69842-313; 0536-1118; 70583-733; 71178-777; 72490-032; 72490-037; 53943-904; 59088-479; 72490-014; 72490-028; 72490-029; 72490-033; 72490-034; 72512-930; 81877-515; 50268-198; 52099-8005; 61787-443; 62742-4216; 63868-308; 71073-208; 71178-776; 73102-117; 73141-559; 73287-018; 75249-003; 17353-0010; 10742-8127; 11993-001; 35192-040; 59088-433; 59779-770; 65923-123; 72490-010; 72490-022; 72490-035; 72490-039; 72683-003; 72683-006; 73486-100; 49452-1321; 51686-0003; 62991-3129; 11993-000; 35192-023; 41250-842; 46122-753; 46581-700; 50268-197; 55264-110; 70000-0556; 71659-842; 72490-036; 73454-001; 82345-855; 59568-9200; 50488-1025; 61787-556; 70656-741; 72490-025; 72490-027; 72490-030; 73557-180; 82777-001; 51824-094; 55614-410; 0363-0837; 67510-0303; 68071-3430; 0536-1264; 71101-904; 72490-019; 81533-200; 51927-1807; 81961-001; 49035-890; 50488-1060; 69396-103; 70000-0549; 72490-012; 72490-013; 72490-021; 72847-316; 82531-001; 51552-0129; 11822-4210; 42903-008; 50268-196; 52000-040 |
| UNII |
S07O44R1ZM
|
| Synonyms |
Capsaicin | Capsaicine | 8-Methyl-N-Vanillyl-6-Nonenamide | 8 Methyl N Vanillyl 6 Nonenamide | Antiphlogistine Rub A-535 Capsaicin | Axsain | Zacin | Capsidol | Zostrix | Capzasin | Gelcen | Katrum | NGX-4010 | NGX 4010 | NGX4010 | Capsicum Farmaya | Capsin |
|
| Chemical Information |
| Molecular Formula |
C18H27NO3 |
| CAS Registry Number |
404-86-4 |
| SMILES |
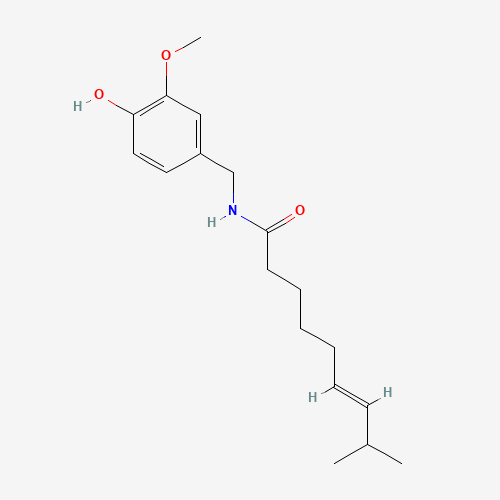
CC(C)C=CCCCCC(=O)NCC1=CC(=C(C=C1)O)OC |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

