| Pharmaceutical Information |
| Drug Name |
Buspirone |
| Drug ID |
BADD_D00316 |
| Description |
Buspirone is a novel anxiolytic agent with a unique structure and a pharmacological profile. Belonging to the azaspirodecanedione drug class,[A180991] buspirone is a serotonin 5-HT1A receptor agonist that is not chemically or pharmacologically related to benzodiazepines, barbiturates, and other sedative/anxiolytic drugs.[L4478] Unlike many drugs used to treat anxiety, buspirone does not exhibit anticonvulsant, sedative, hypnotic, and muscle-relaxant properties. Due to these characteristics, buspirone been termed 'anxioselective'.[A180985] First synthesized in 1968 then patented in 1975,[L7375] it is commonly marketed under the brand name Buspar®. Buspirone was first approved in 1986 by the FDA [A181751] and has been used to treat anxiety disorders, such as generalized anxiety disorder (GAD), and relieve symptoms of anxiety. It has also been used as a second-line therapy for unipolar depression when the use of selective serotonin reuptake inhibitors (SSRIs) is deemed clinically inadequate or inappropriate.[L7375] The potential use of buspirone in combination with [melatonin] in depression and cognitive impairment via promoting neurogenesis has also been investigated.[A181751] |
| Indications and Usage |
Indicated for the management of anxiety disorders or the short-term relief of the symptoms of anxiety.[L4478] |
| Marketing Status |
approved; investigational |
| ATC Code |
N05BE01 |
| DrugBank ID |
DB00490
|
| KEGG ID |
D07593
|
| MeSH ID |
D002065
|
| PubChem ID |
2477
|
| TTD Drug ID |
D0U2OO
|
| NDC Product Code |
Not Available |
| UNII |
TK65WKS8HL
|
| Synonyms |
Buspirone | N-(4-(4-(2-pyrimidinyl)-1-piperazinyl)butyl)-1-cyclopentanediacetamide | Buspirone Hydrochloride | Hydrochloride, Buspirone | Gen-Buspirone | Gen Buspirone | Lin-Buspirone | Lin Buspirone | MJ-9022-1 | MJ 9022 1 | MJ90221 | Neurosine | Busp | Nu-Buspirone | Nu Buspirone | PMS-Buspirone | PMS Buspirone | Ratio-Buspirone | Ratio Buspirone | Anxut | Apo-Buspirone | Apo Buspirone | Buspar | Bespar | Novo-Buspirone | Novo Buspirone |
|
| Chemical Information |
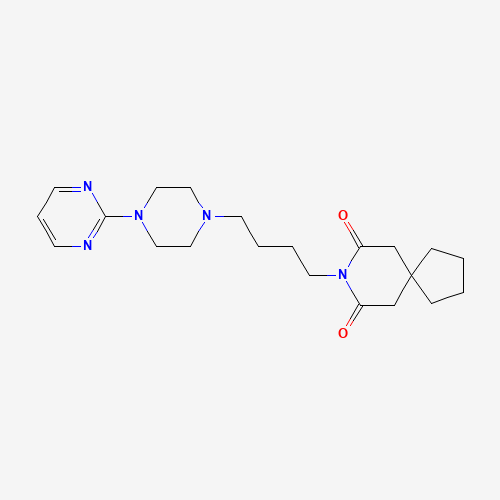
| Molecular Formula |
C21H31N5O2 |
| CAS Registry Number |
36505-84-7 |
| SMILES |
C1CCC2(C1)CC(=O)N(C(=O)C2)CCCCN3CCN(CC3)C4=NC=CC=N4 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

