| Pharmaceutical Information |
| Drug Name |
Azathioprine |
| Drug ID |
BADD_D00195 |
| Description |
Azathioprine is a prodrug of 6-mercaptopurine, first synthesized in 1956 by Gertrude Elion, William Lange, and George Hitchings in an attempt to produce a derivative of 6-mercaptopurine with a better therapeutic index.[A189678,A189687,A189690] Azathioprine is used to treat inflammatory conditions like rheumatoid arthritis and as an immunosuppressant in the prevention of renal transplant rejection.[L11214]
Azathiprine was granted FDA approval on 20 March 1968.[L11214] |
| Indications and Usage |
Azathioprine is indicated to treat rheumatoid arthritis and prevent renal transplant rejection.[L11214] |
| Marketing Status |
approved |
| ATC Code |
L04AX01 |
| DrugBank ID |
DB00993
|
| KEGG ID |
D00238
|
| MeSH ID |
D001379
|
| PubChem ID |
2265
|
| TTD Drug ID |
D07QCE
|
| NDC Product Code |
62991-2189; 65649-231; 67877-492; 68084-229; 68382-120; 51407-182; 68382-118; 72789-129; 46014-1110; 70771-1141; 60219-1076; 60219-2036; 60219-2037; 68382-003; 48954-909; 51927-0071; 68682-241; 70518-3544; 70771-1140; 51927-2258; 54766-590; 65841-602; 12780-0300; 38779-0312; 66122-0009; 42291-063; 68462-502; 71610-124; 42291-071; 68682-231; 70771-1139; 15308-0732; 49452-0783; 67877-493; 51552-0779; 65649-241; 67877-494; 67877-495; 68382-119; 42973-143 |
| UNII |
MRK240IY2L
|
| Synonyms |
Azathioprine | Azothioprine | Imurel | Imuran | Immuran | Azathioprine Sodium | Sodium, Azathioprine | Azathioprine Sodium Salt | Azathioprine Sulfate |
|
| Chemical Information |
| Molecular Formula |
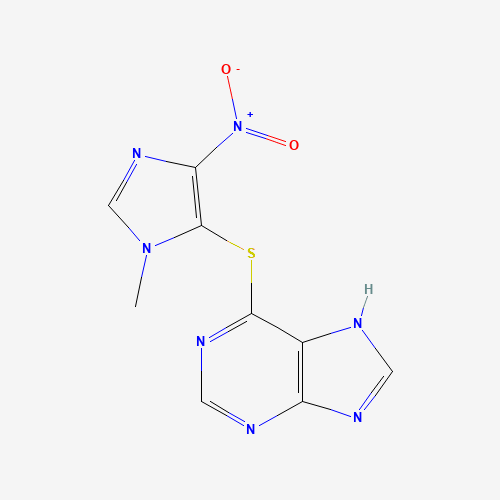
C9H7N7O2S |
| CAS Registry Number |
446-86-6 |
| SMILES |
CN1C=NC(=C1SC2=NC=NC3=C2NC=N3)[N+](=O)[O-] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
|
|
|

