| Pharmaceutical Information |
| Drug Name |
Axitinib |
| Drug ID |
BADD_D00193 |
| Description |
Axitinib is a second generation tyrosine kinase inhibitor that works by selectively inhibiting vascular endothelial growth factor receptors (VEGFR-1, VEGFR-2, VEGFR-3).[L6676] Through this mechanism of action, axitinib blocks angiogenesis, tumour growth and metastases. It is reported to exhibit potency that is 50-450 times higher than that of the first generation VEGFR inhibitors.[L6676] Axitinib is an indazole derivative.[A179398] It is most commonly marketed under the name Inlyta® and is available in oral formulations. |
| Indications and Usage |
Used in kidney cell cancer and investigated for use/treatment in pancreatic and thyroid cancer. |
| Marketing Status |
approved; investigational |
| ATC Code |
L01EK01 |
| DrugBank ID |
DB06626
|
| KEGG ID |
D03218
|
| MeSH ID |
D000077784
|
| PubChem ID |
6450551
|
| TTD Drug ID |
D01ZRI
|
| NDC Product Code |
0069-0145; 54893-0032; 68554-0088; 63539-026; 82920-028; 0069-0151; 65344-0033; 63539-044; 54893-0043 |
| UNII |
C9LVQ0YUXG
|
| Synonyms |
Axitinib | AG 013736 | AG013736 | AG-013736 | Inlyta |
|
| Chemical Information |
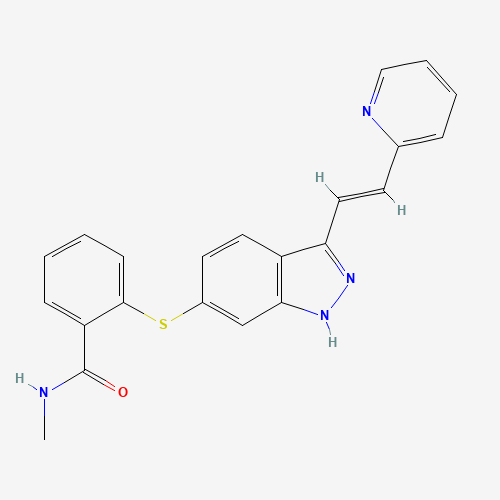
| Molecular Formula |
C22H18N4OS |
| CAS Registry Number |
319460-85-0 |
| SMILES |
CNC(=O)C1=CC=CC=C1SC2=CC3=C(C=C2)C(=NN3)C=CC4=CC=CC=N4 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
|
|
|

