| Pharmaceutical Information |
| Drug Name |
Apremilast |
| Drug ID |
BADD_D00156 |
| Description |
Apremilast, also known as Otezla, is a phosphodiesterase 4 (PDE4) inhibitor used to treat various types of symptoms resulting from certain inflammatory autoimmune diseases. It belongs to the same drug class as [Roflumilast] and [Crisaborole].[A181244,L7495] Initially approved in 2014, it is marketed by Celgene.[L7501] In July 2019, apremilast was granted a new FDA approval for the treatment of oral ulcers associated with Behcet's disease, an autoimmune condition that causes recurrent skin, blood vessel, and central nervous system inflammation.[A181216] |
| Indications and Usage |
Apremilast is indicated for the treatment of adults with active psoriatic arthritis and adults with oral ulcers associated with Behcet's Disease. In addition, apremilast is indicated for the treatment of plaque psoriasis, of any severity, in adult patients who are candidates for phototherapy or systemic therapy.[L7501] |
| Marketing Status |
approved; investigational |
| ATC Code |
L04AA32 |
| DrugBank ID |
DB05676
|
| KEGG ID |
D08860
|
| MeSH ID |
C505730
|
| PubChem ID |
10151715
|
| TTD Drug ID |
D07ESC
|
| NDC Product Code |
55513-369; 53069-1040; 66406-0242; 82891-014; 55513-137; 59651-150; 65129-1383; 11722-062; 12658-0581; 47621-306; 53069-1050; 66406-0241; 70518-3155; 49187-0757; 55111-993; 58032-2030; 65372-1204; 69766-010; 69766-024; 55513-485; 53747-077; 65977-0119; 66039-934; 73309-043; 66406-0240 |
| UNII |
UP7QBP99PN
|
| Synonyms |
apremilast | Otezla | CC 10004 | CC10004 | CC-10004 |
|
| Chemical Information |
| Molecular Formula |
C22H24N2O7S |
| CAS Registry Number |
253168-86-4 |
| SMILES |
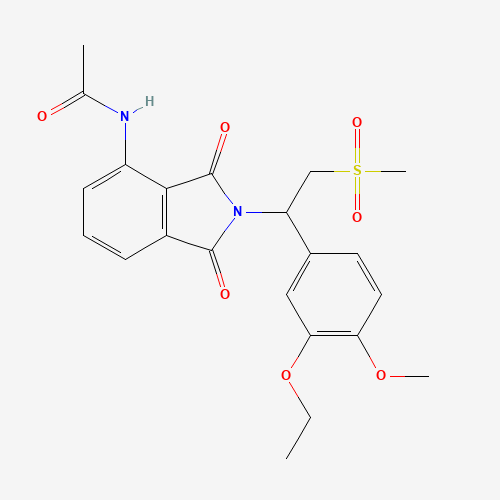
CCOC1=C(C=CC(=C1)C(CS(=O)(=O)C)N2C(=O)C3=C(C2=O)C(=CC=C3)NC(=O)C)OC |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
|
|
|

