| Pharmaceutical Information |
| Drug Name |
Amoxicillin |
| Drug ID |
BADD_D00129 |
| Description |
Amoxicillin, or BRL-2333, is a penicillin G derivative first described in the literature in 1972.[A190648] Amoxicillin has similar activity to [penicillin] and [ampicillin], but leads to higher serum concentrations than ampicillin.[A190648]
Amoxicillin was granted FDA approval on 18 January 1974.[L11644] |
| Indications and Usage |
Amoxicillin alone is indicated to treat susceptible bacterial infections of the ear, nose, throat, genitourinary tract, skin, skin structure, and lower respiratory tract.[L11656,L11659] Amoxicillin is given with calvulanic acid to treat acute bacterial sinusitis, community acquired pneumonia, lower respiratory tract infections, acute bacterial otitis media, skin and skin structure infections, and urinary tract infections.[L11650,L7880,L11653] Amoxicillin is given with omeprazole in the treatment of _H. pylori_.[L9743,L11647] |
| Marketing Status |
approved; vet_approved |
| ATC Code |
J01CA04 |
| DrugBank ID |
DB01060
|
| KEGG ID |
D00229; D07452
|
| MeSH ID |
D000658
|
| PubChem ID |
33613
|
| TTD Drug ID |
D0F6EO
|
| NDC Product Code |
0781-5060; 55657-101; 55657-106; 59651-099; 65862-274; 0093-2268; 0093-4161; 43063-563; 0143-9886; 50090-0304; 50090-0858; 50090-1511; 50090-5863; 50090-5880; 51655-156; 53002-0130; 53002-2080; 55289-182; 57237-029; 61919-672; 61919-716; 63187-200; 63187-591; 68071-1760; 68071-2908; 68071-4548; 68788-8240; 70518-0895; 72789-224; 76420-192; 81964-225; 43063-820; 50090-0713; 50090-6056; 50090-6466; 53002-1369; 55289-019; 57237-028; 61919-401; 63187-044; 63187-295; 65862-071; 65862-706; 67296-1156; 68071-4455; 68788-7318; 68788-9948; 70518-2188; 70518-2190; 70518-3294; 71205-177; 71205-436; 76420-112; 76420-508; 0781-6039; 0093-4160; 0143-9285; 43063-341; 63187-262; 63629-7587; 63629-7798; 63629-7801; 68071-2766; 68071-2875; 68071-3119; 68788-6374; 68999-825; 55289-020; 63187-043; 63187-186; 63629-7633; 66267-994; 67296-1894; 68071-4693; 68071-4741; 68788-8324; 71205-035; 71205-422; 0781-6041; 16714-299; 0093-2267; 42571-234; 42708-153; 43063-574; 50090-0288; 50090-1512; 50090-1812; 50090-6351; 53002-2369; 63187-027; 67296-0986; 68071-2558; 68788-8209; 68788-8304; 68788-8325; 68788-9018; 71205-507; 76420-003; 52115-001; 65862-014; 65862-017; 50090-0406; 50090-0407; 50090-6469; 53002-2160; 57237-032; 63187-325; 68071-4953; 70518-2191; 70882-113; 70934-038; 76420-545; 16714-298; 0093-2264; 42708-010; 42708-076; 0143-9888; 0143-9939; 50090-1810; 53002-2090; 63187-029; 63187-399; 63187-880; 65862-707; 68071-2604; 70518-3234; 70882-114; 71205-266; 72789-254; 0781-2613; 0781-5061; 0781-6156; 42708-112; 43063-885; 50090-0301; 50090-0404; 50090-1086; 50090-1347; 61919-135; 63187-013; 63187-335; 63187-343; 63187-729; 65862-015; 65862-016; 65862-070; 68071-2320; 68071-2911; 70518-3228; 76420-573; 80425-0269; 0093-3109; 0093-4155; 50090-1513; 50090-1813; 50090-2251; 50090-6349; 57237-031; 61919-994; 63629-7585; 67296-1126; 68071-1826; 68788-6369; 68788-7323; 68788-8352; 68788-9019; 72189-463; 72789-225; 0781-6157; 81964-205; 83112-031; 0093-2263; 42571-233; 0143-9887; 50090-1826; 50090-6319; 50090-6355; 50436-0031; 53002-2091; 57237-033; 68071-3235; 68788-7414; 68788-9017; 68788-9949; 70934-064; 71205-775; 72189-285; 0781-2020; 76420-138; 82982-022; 0093-3107; 42708-078; 43063-915; 0143-9889; 50090-0302; 50090-0303; 50090-0405; 50090-0709; 50090-1811; 50090-6323; 57237-030; 59115-041 |
| UNII |
804826J2HU
|
| Synonyms |
Amoxicillin | Amoxycillin | Amoxicilline | Amoxicillin Monopotassium Salt | Hydroxyampicillin | Amoxicillin Sodium | Amoxicillin Monosodium Salt | Amoxicillin, (R*)-Isomer | Amoxicillin Anhydrous | Actimoxi | BRL-2333 | BRL 2333 | BRL2333 | Clamoxyl | Penamox | Clamoxyl Parenteral | Clamoxyl G.A. | Polymox | Trimox | Wymox | Amoxicillin Trihydrate | Amoxil |
|
| Chemical Information |
| Molecular Formula |
C16H19N3O5S |
| CAS Registry Number |
26787-78-0 |
| SMILES |
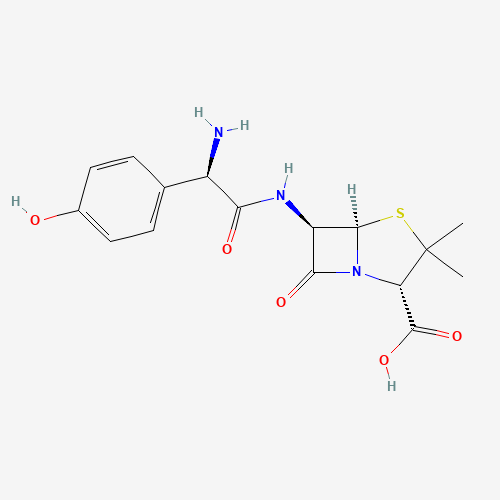
CC1(C(N2C(S1)C(C2=O)NC(=O)C(C3=CC=C(C=C3)O)N)C(=O)O)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
|
|
|

