| Pharmaceutical Information |
| Drug Name |
Aminosalicylic acid |
| Drug ID |
BADD_D00114 |
| Description |
An antitubercular agent often administered in association with isoniazid. The sodium salt of the drug is better tolerated than the free acid. |
| Indications and Usage |
For the treatment of tuberculosis |
| Marketing Status |
approved |
| ATC Code |
J04AA01 |
| DrugBank ID |
DB00233
|
| KEGG ID |
D00162
|
| MeSH ID |
D010131
|
| PubChem ID |
4649
|
| TTD Drug ID |
D01WJL
|
| NDC Product Code |
76003-0710 |
| UNII |
5B2658E0N2
|
| Synonyms |
Aminosalicylic Acid | Acid, Aminosalicylic | 4-Aminosalicylic Acid | 4 Aminosalicylic Acid | para-Aminosalicylic Acid | para Aminosalicylic Acid | p-Aminosalicylic Acid | p Aminosalicylic Acid | p-Aminosalicylic Acid Monolithium Salt | p Aminosalicylic Acid Monolithium Salt | p-Aminosalicylic Acid Monopotassium Salt | p Aminosalicylic Acid Monopotassium Salt | Rezipas | p-Aminosalicylic Acid, Aluminum (2:1) Salt | p-Aminosalicylic Acid, Calcium (2:1) Salt | p-Aminosalicylic Acid, Monosodium Salt, Dihydrate | Pamisyl | Alumino-4-Aminosalicylic Acid | Alumino 4 Aminosalicylic Acid | p-Aminosalicylic Acid Monosodium Salt | p Aminosalicylic Acid Monosodium Salt |
|
| Chemical Information |
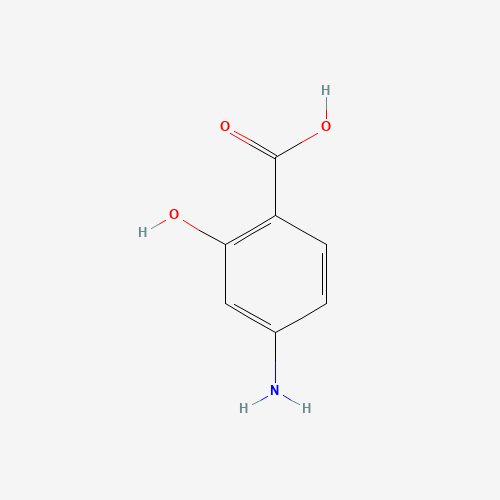
| Molecular Formula |
C7H7NO3 |
| CAS Registry Number |
65-49-6 |
| SMILES |
C1=CC(=C(C=C1N)O)C(=O)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

