| Pharmaceutical Information |
| Drug Name |
Allopurinol |
| Drug ID |
BADD_D00076 |
| Description |
Gout is a disease that occurs by the deposition of monosodium urate crystals (MSU) in body tissues, especially around joints [A175942]. This disease has been well-documented in historical medical records and appears in the biographies of several prominent, historically recognized individuals [A175942].
Allopurinol is a xanthine oxidase enzyme inhibitor that is considered to be one of the most effective drugs used to decrease urate levels and is frequently used in the treatment of chronic gout [A36705]. It was initially approved by the FDA in 1966 [L5674] and is now formulated by several manufacturers [L5677]. |
| Indications and Usage |
Allopurinol is indicated in [FDA label]:
1) the management of patients with signs and symptoms of primary or secondary gout (acute attacks, tophi, joint destruction, uric acid lithiasis, and/or nephropathy).
2) the management of patients with leukemia, lymphoma and malignancies who are receiving cancer therapy which causes elevations of serum and urinary uric acid levels. Treatment with allopurinol should be discontinued when the potential for overproduction of uric acid is no longer present.
3) the management of patients with recurrent calcium oxalate calculi whose daily uric acid excretion exceeds 800 mg/day in male patients and 750 mg/day in female patients. Therapy in such patients should be carefully assessed initially and reassessed periodically to determine in each case that treatment is beneficial and that the benefits outweigh the risks. |
| Marketing Status |
approved |
| ATC Code |
M04AA01 |
| DrugBank ID |
DB00437
|
| KEGG ID |
D00224
|
| MeSH ID |
D000493
|
| PubChem ID |
135401907
|
| TTD Drug ID |
D04KYY
|
| NDC Product Code |
23155-694; 43063-975; 55154-5534; 60687-688; 62135-516; 66064-1043; 69967-009; 70710-1209; 70934-681; 71610-676; 72189-437; 43063-934; 55154-5454; 58118-1156; 60687-677; 60760-656; 61919-748; 63187-463; 0378-0181; 67296-1314; 68083-380; 68788-8364; 70199-031; 71610-194; 0603-2116; 51927-0070; 51927-1915; 16571-885; 16714-042; 16729-135; 43353-186; 51655-082; 0378-0137; 66267-665; 67544-313; 68071-2997; 68788-8458; 69967-008; 70199-016; 70518-3660; 70710-1210; 0603-2115; 71610-678; 63629-2113; 63739-410; 66064-1044; 68788-6323; 71610-681; 63552-151; 68108-0212; 51079-206; 51655-968; 53002-4821; 53489-157; 55111-729; 63739-796; 68071-2616; 68071-2660; 68788-7383; 70934-882; 71205-208; 71610-064; 0591-5544; 49452-0008; 65015-606; 66064-2100; 16571-883; 16714-041; 43353-501; 51655-523; 51655-782; 53002-4820; 55700-947; 62584-988; 63187-240; 63629-2112; 70771-1126; 0591-5543; 71610-253; 0615-8385; 57451-1166; 16714-576; 23155-693; 43063-976; 51655-945; 55154-7981; 60760-139; 62584-713; 63629-7908; 67457-187; 67457-978; 67544-736; 70199-033; 71205-377; 72162-1150; 0904-7041; 17351-0041; 36974-0018; 16571-884; 29300-349; 50090-5168; 51079-205; 61919-471; 62135-517; 63629-2111; 63629-8020; 65219-380; 67296-1265; 69315-291; 70518-1806; 70518-3289; 70771-1127; 70934-979; 71335-0467; 71335-1656; 71335-9634; 71610-197; 72189-417; 80425-0206; 0904-6572; 29300-350; 50090-4662; 68071-2926; 69315-292; 70199-032; 70518-3704; 71205-049; 71335-9641; 71335-9676; 72162-1149; 49711-0113; 63552-150; 65977-0124; 16714-577; 16729-134; 43063-935; 53489-156; 55700-935; 60760-134; 68788-8337; 68788-8424; 70199-015; 70518-0451; 71335-0112; 14445-001; 72375-0007; 50090-0044; 50090-5194; 55111-730; 55154-2338 |
| UNII |
63CZ7GJN5I
|
| Synonyms |
Allopurinol | Uribenz | Allopurin | Allorin | Allpargin | Allural | Pan Quimica | Apulonga | Apurin | Atisuril | Bleminol | Caplenal | Capurate | Cellidrin | Embarin | Suspendol | Foligan | Hamarin | Lopurin | Lysuron | Jenapurinol | Milurit | Milurite | Novopurol | Uripurinol | Urosin | Urtias | Xanthomax | Uridocid | Xanturic | Zygout | Zyloprim | Zyloric | Pureduct | Purinol | Progout | Remid | Rimapurinol | Roucol | Tipuric | Allohexal | Allohexan | Alloprin |
|
| Chemical Information |
| Molecular Formula |
C5H4N4O |
| CAS Registry Number |
315-30-0 |
| SMILES |
C1=NNC2=C1C(=O)NC=N2 |
| Chemical Structure |
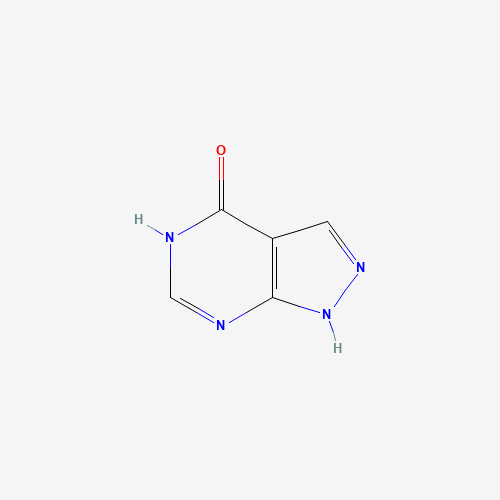
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
|
|
|

