| Pharmaceutical Information |
| Drug Name |
Afatinib |
| Drug ID |
BADD_D00047 |
| Description |
Afatinib is a 4-anilinoquinazoline tyrosine kinase inhibitor in the form of a dimaleate salt available as Boehringer Ingelheim's brand name Gilotrif [FDA Label]. For oral use, afatinib tablets are a first-line (initial) treatment for patients with metastatic non-small cell lung cancer (NSCLC) with common epidermal growth factor receptor (EGFR) mutations as detected by an FDA-approved test [L2939]. Gilotrif (afatinib) is the first FDA-approved oncology product from Boehringer Ingelheim [L2939]. |
| Indications and Usage |
Afatinib is a kinase inhibitor indicated as monotherapy [L2937] for the first-line [FDA Label] treatment of (a) Epidermal Growth Factor Receptor (EGFR) TKI (tyrosine kinase inhibitor)-naive adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) whose tumours have non-resistant EGFR mutations as detected by an FDA-approved test [FDA Label], and (b) adult patients with locally advanced or metastatic NSCLC of squamous histology progressing on or after platinum-based chemotherapy [FDA Label, L2937].
Recently, as of January 2018, the US FDA approved a supplemental New Drug Application for Boehringer Ingelheim's Gilotrif (afatinib) for the first line treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have non-resistant epidermal growth factor receptor (EGFR) mutations as detected by an FDA-approved test [L2939]. The new label includes data on three additional EGFR mutations: L861Q, G719X and S768I [L2939]. |
| Marketing Status |
approved |
| ATC Code |
L01EB03 |
| DrugBank ID |
DB08916
|
| KEGG ID |
D09724
|
| MeSH ID |
D000077716
|
| PubChem ID |
10184653
|
| TTD Drug ID |
D05UFG
|
| NDC Product Code |
0597-0137; 0597-0138; 0597-0141 |
| UNII |
41UD74L59M
|
| Synonyms |
Afatinib | (2E)-N-(4-(3-Chloro-4-fluoroanilino)-7-(((3S)-oxolan-3-yl)oxy)quinoxazolin-6-yl)-4-(dimethylamino)but-2-enamide | BIBW-2992-MA2 | BIBW 2992 MA2 | BIBW-2992MA2 | BIBW 2992MA2 | BIBW2992 MA2 | Afatinib Maleate | BIBW 2992 | BIBW2992 | BIBW-2992 | Gilotrif | Afatinib Dimaleate |
|
| Chemical Information |
| Molecular Formula |
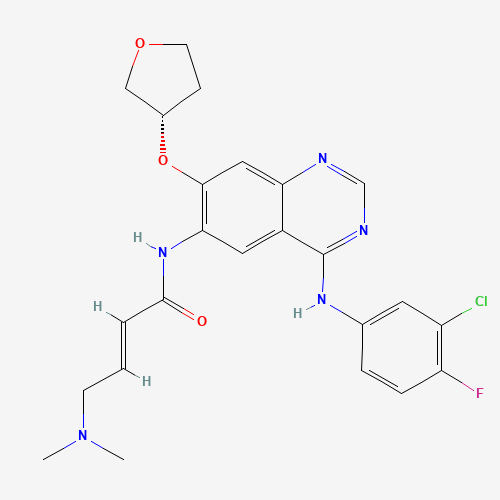
C24H25ClFN5O3 |
| CAS Registry Number |
850140-72-6 |
| SMILES |
CN(C)CC=CC(=O)NC1=C(C=C2C(=C1)C(=NC=N2)NC3=CC(=C(C=C3)F)Cl)OC4CCOC4 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
|
|
|

