| Pharmaceutical Information |
| Drug Name |
Acetylcholine |
| Drug ID |
BADD_D00030 |
| Description |
A neurotransmitter. Acetylcholine in vertebrates is the major transmitter at neuromuscular junctions, autonomic ganglia, parasympathetic effector junctions, a subset of sympathetic effector junctions, and at many sites in the central nervous system. It is generally not used as an administered drug because it is broken down very rapidly by cholinesterases, but it is useful in some ophthalmological applications. |
| Indications and Usage |
Used to obtain miosis of the iris in seconds after delivery of the lens in cataract surgery, in penetrating keratoplasty, iridectomy and other anterior segment surgery where rapid miosis may be required. |
| Marketing Status |
approved; investigational |
| ATC Code |
S01EB09 |
| DrugBank ID |
DB03128
|
| KEGG ID |
D00999
|
| MeSH ID |
D000109
|
| PubChem ID |
187
|
| TTD Drug ID |
D0Q9HF
|
| NDC Product Code |
Not Available |
| UNII |
N9YNS0M02X
|
| Synonyms |
Acetylcholine | 2-(Acetyloxy)-N,N,N-trimethylethanaminium | Acetylcholine L-Tartrate | Acetylcholine L Tartrate | L-Tartrate, Acetylcholine | Acetylcholine Perchlorate | Perchlorate, Acetylcholine | Acetylcholine Picrate (1:1) | Acetylcholine Picrate | Acetylcholine Hydroxide | Hydroxide, Acetylcholine | Miochol | Acetilcolina Cusi | Cusi, Acetilcolina | Acetylcholine Bromide | Bromide, Acetylcholine | Bromoacetylcholine | Acetylcholine Chloride | Chloroacetylcholine | Acetylcholine Fluoride | Fluoride, Acetylcholine | Acetylcholine Iodide | Iodide, Acetylcholine | Acetylcholine Sulfate (1:1) |
|
| Chemical Information |
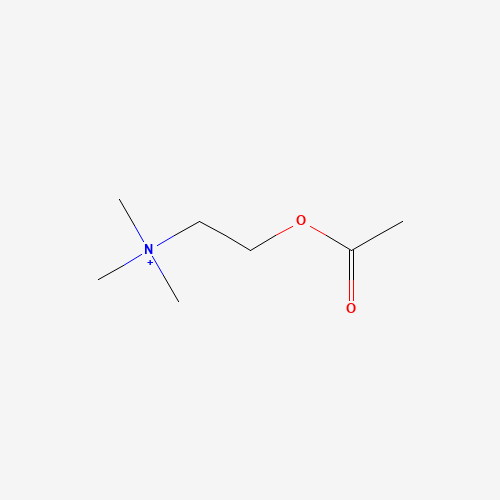
| Molecular Formula |
C7H16NO2+ |
| CAS Registry Number |
51-84-3 |
| SMILES |
CC(=O)OCC[N+](C)(C)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

