| Pharmaceutical Information |
| Drug Name |
Aceclofenac |
| Drug ID |
BADD_D00021 |
| Description |
Aceclofenac is an oral non-steroidal anti-inflammatory drug (NSAID) with marked anti-inflammatory and analgesic properties used to treat osteoarthritis, rheumatoid arthritis and ankylosing spondylitis. It is reported to have a higher anti-inflammatory action or at least comparable effects than conventional NSAIDs in double-blind studies [A19667, A19668, A19670]. Aceclofenac potently inhibits the cyclo-oxygenase enzyme (COX) that is involved in the synthesis of prostaglandins, which are inflammatory mediators that cause pain, swelling, inflammation, and fever. Aceclofenac belongs to BCS Class II as it possesses poor aqueous solubility [A19667]. It displays high permeability to penetrate into synovial joints where in patients with osteoarthritis and related conditions, the loss of articular cartilage in the area causes joint pain, tenderness, stiffness, crepitus, and local inflammation [A19666]. Aceclofenac is also reported to be effective in other painful conditions such as dental and gynaecological conditions [A19672]. In 1991, aceclofenac was developed as an analog of a commonly prescribed NSAID, [DB00586], via chemical modification in effort to improve the gastrointestinal tolerability of the drug. It is a more commonly prescribed drug in Europe. |
| Indications and Usage |
Aceclofenac is indicated for the relief of pain and inflammation in osteoarthritis, rheumatoid arthritis and ankylosing spondylitis. |
| Marketing Status |
approved; investigational |
| ATC Code |
M01AB16; M02AA25 |
| DrugBank ID |
DB06736
|
| KEGG ID |
D01545
|
| MeSH ID |
C056498
|
| PubChem ID |
71771
|
| TTD Drug ID |
D0T8VY
|
| NDC Product Code |
Not Available |
| UNII |
RPK779R03H
|
| Synonyms |
aceclofenac | 2-((2,6-dichlorophenyl)amino)phenylacetoxyacetic acid | Falcol | Falcol Difucrem | Clanza CR | Preservex | Biofenac | Sanein | Beofenac | Aital | Airtal Difucrem | Airtal | Bristaflam | Gerbin | Gerbin Difucrem |
|
| Chemical Information |
| Molecular Formula |
C16H13Cl2NO4 |
| CAS Registry Number |
89796-99-6 |
| SMILES |
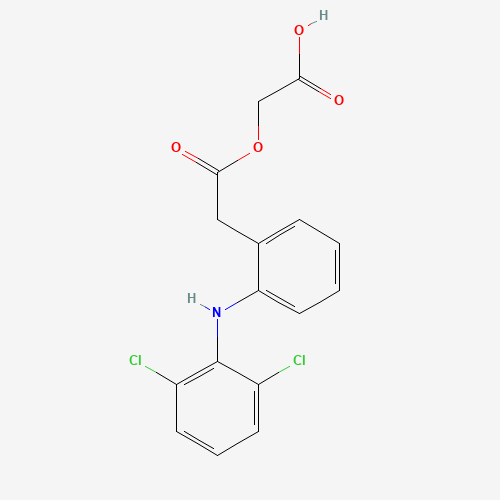
C1=CC=C(C(=C1)CC(=O)OCC(=O)O)NC2=C(C=CC=C2Cl)Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Abdominal pain | 07.01.05.002 | - | - | | | Dizziness | 02.11.04.006; 24.06.02.007; 17.02.05.003 | - | - | | | Dyspepsia | 07.01.02.001 | - | - | | | Enuresis | 20.02.02.003; 19.07.04.001 | - | - | Not Available | | Headache | 17.14.01.001 | - | - | | | Nausea | 07.01.07.001 | - | - | | | Pain | 08.01.08.004 | - | - | | | Rash | 23.03.13.001 | - | - | Not Available | | Rubella | 11.05.18.001 | - | - | Not Available | | Somnolence | 19.02.05.003; 17.02.04.006 | - | - | | | Urticaria | 23.04.02.001; 10.01.06.001 | - | - | |
|
The 1th Page
1
Total 1 Pages
|
|

